Effects of antibiotic sulfonamides on soil microbial population and respiration in rhizospheric soil of wheat
A group from Molecular Plant Physiology, Institute of Botany, University of the Punjab, Lahore, Pakistan, etc. has reported about the effects of sulfonamides on soil microbial population and respiration in rhizospheric soil of wheat.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0264476
Sulfonamide is widely used in livestock farming and has been used to treat a variety of bacterial diseases. Due to poor management, they are excreted into the soil after treatments and these are extremely hazardous. Sulfonamides may impair the growth of plants, leaves, and roots at concentrations of several hundred mg/L. Accumulations of different antibiotics, including sulfonamides harmed the function and activity of microorganism and reduce soil enzymatic activity. Antibiotics in soil can bring constant changes in organisms and plants and exert harmful impacts on soil microbes.
This study was conducted to infer impact of four newly synthesized sulfonamides on isolated native strains from rhizosphere of wheat cultivar ‘Chakwal-50’. Furthermore, present research focused on the susceptibility of soil microbes and microbial respiration in the rhizospheric soil of wheat.
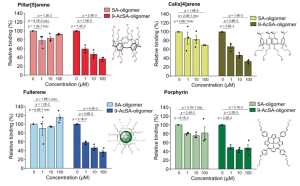
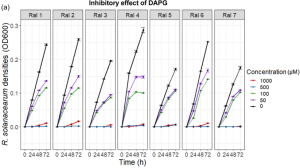
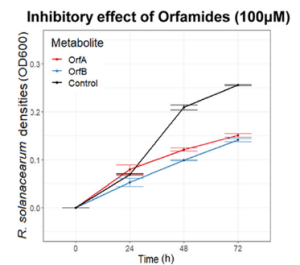
Sulfonamides: 2-(phenylsulfonyl) hydrazine carbothioamide (TSBS-1), N, 2-bis phenyl hydrazine carbothioamide (TSBS-2), aminocarbonyl benzene sulfonamide (UBS-1), and N, N’-carbonyl dibenzene sulfonamide (UBS-2) were applied on five isolated bacterial strains, i.e., AC (Actinobacter spp), RS-3a (Bacillus spp.), RS-7a (Bacillus subtilis), RS-4a (Enterobacter spp.) and RS-5a (Enterobacter spp.) isolated from the wheat rhizosphere. All sulfonamide derivatives exhibited antibacterial activity against tested bacterial strains, except for TSBS-1. In comparison of all sulfonamide derivatives, UBS-1 exhibited the highest inhibition zone (11.47 ± 0.90 mm) against RS-4a at the highest concentration (4 mg/ml).
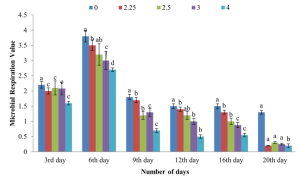 numbers in the figure are UBS-1 concentrations (mg/mL)
numbers in the figure are UBS-1 concentrations (mg/mL)
Thus, sulfonamides have a negative influence on the soil microbiome, and some soil microorganisms that are unable to resist such stressors, so it is difficult to retain soil fertility and plant development as well. Soil microbial respiration changes mediated by sulfonamides were dependent on length of exposure and concentration. It is needed that antibiotics should be carefully watched and their impact on plant growth should be tested in the future studies.