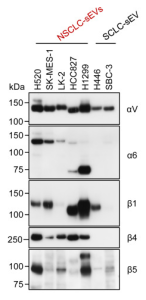
Plant Natural Biopesticides: natural antimicrobial volatile organic compounds produced by entomopathogenic fungi
A group from Faculty of Science and Engineering, Swansea University, Singleton Park, Swansea SA2 8PP, Wales, UK, etc. has reported that natural antimicrobial volatile organic compounds (VOCs) produced by entomopathogenic fungi (EPF) have have the potential for use as plant natural biopesticides.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9025432/
EPF form symbiotic relationships with plants, leading to improved plant growth and productivity. Plants treated with EPF species, such as Metarhizium brunneum and Beauveria bassiana, usually have more extensive root systems, greater biomass and higher crop yields than untreated plants.
In this study, antimicrobial properties of fungal VOCs produced by these EPF were investigated against the following soil microbes,
Gram-negative bacteria (Escherichia coli, Pantoea agglomerans, Pseudomonas aeruginosa),
Gram-positive bacteria (Micrococcus luteus, Staphylococcus aureus, Bacillus subtilis, B. megaterium, B. thuringiensis),
Yeasts (Candida albicans, Candida glabrata), and
Plant pathogenic fungi (Pythium ultimum, Botrytis cinerea, Fusarium graminearum).
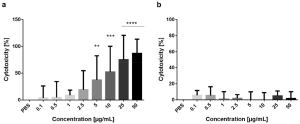
The most potent antimicrobials were isovaleric acid and 1-octen-3-ol, which inhibited or killed bacteria, yeasts, filamentous fungi and the oomycete P. ultimum. In contrast, isoamyl formate, 3-octenone, cedrene and farnesene, although potent, were more restricted in their specificity. Some microbes were clearly more sensitive to M. brunneum VOCs than others. In order of sensitivity, F. graminearum was inhibited by far more compounds than any other test microbe, followed by B. cinerea, then P. ultimum. Of the test bacteria, B. megaterium was the most sensitive, followed by B. thuringiensis.
The natural antimicrobial VOCs identified in this study have the potential for use as plant natural biopesticides.