Dual inhibition of TMPRSS2 and Cathepsin B is effective in preventing SARS-CoV-2 infection in ACE2-iPS cells
A group from Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan has reported that dual inhibition of TMPRSS2 and Cathepsin B is effective in preventing SARS-CoV-2 infection.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8527102/pdf/main.pdf
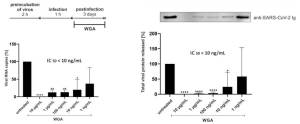
iPS cells that stably express ACE2 were used for this experiment, and TMPRSS2 and CTSB (Cathepsin B gene) were suppressed to about 1% or less, by using a CRISPER interference system.
It was found that CTSB and TMPRSS2 are required for SARS-CoV-2 to infect ACE2-iPS cells. It is known that TMPRSS2 is present in the cell membrane and CTSB in endosomes, suggesting TMPRSS2 and CTSB play important roles in endocytosis independent and endocytosis-dependent infection, respectively.
From this experiment, it was shown that Double-knockdown of TMPRSS2 and CTSB reduced the viral load to 0.036±0.021%.