A group from Department of Chemistry, University of Alberta, Edmonton, AB, Canada, etc. has reported that α2-6Sia and its underlying enzyme ST6GAL1 as a potentially important glycan epitope in human Pancreatic ductal adenocarcinoma (PDAC).
https://www.mcponline.org/article/S1535-9476(21)00132-8/fulltext
Lectin Microarray:
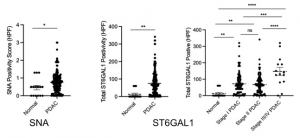
In PDAC, significant increases were observed in sialic acids, with changes in both α2-6 (lectins: SNA, TJA-I, PSL, average increase: ~ 2.7 fold) and α2-3sialosides (diCBM40, SLBR-H, SLBR-B, SLBR-N, MAL-I, MAA, ~ 3.4 fold).
Core fucosylation (PSA, LcH, ~ 2.7 fold) and bisecting GlcNAc (PHA-E, ~ 2.2 fold) and poly-LacNAc (WGA, DSA, LEA, ∼ 2.1 fold) also increased relative to normal.
Glycosyltransferases:
Strong ST6GAL1 expression was observed in endothelial, immune cell and the cancerous ductal clusters. In addition, ST6GAL1 levels were associated with reduced survival. The α2-3 sialyltransferase ST3GAL1 was also observed in many cell types, however it was not enriched in cancer-specific ductal cells.