Monoclonal antibody reference standard with extensive glycan characterization: NISTmAb
Global Biologics, Science Division, United States Pharmacopeia, Rockville, MD 20852, USA has disclosed a monoclonal antibody reference standard, with extensive glycan characterization.
https://www.mdpi.com/1424-8247/15/3/315
A monoclonal antibody reference standard, with extensive glycan characterization is available from the National Institute of Standards and Technology designated as NIST Reference Material 8671, NISTmAb.
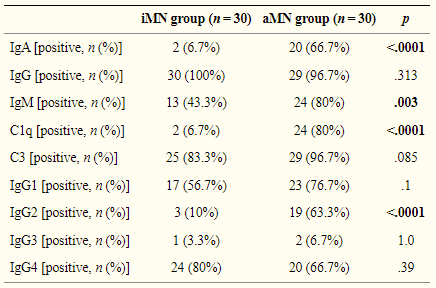
The United States Pharmacopeia (USP) developed three monoclonal antibody reference standards (i.e., USP mAb 001 RS, USP mAb 002 RS, and USP mAb 003 RS) that may be used as control materials to demonstrate whether glycan characterization procedures provide an accurate result.
The USP mAb reference standards are different proteins of the same IgG type 1 subclass yet provide sufficient variability to examine a broad spectrum of glycan structures. Glycan characterization is commonly performed by analysis of glycans released from the protein backbone.
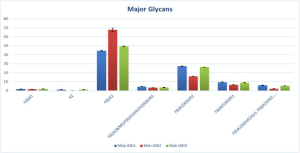
where,
F(6)A2: core fucosylated biantennary,
F(6)A2B: core fucosylated biantennary with bisecting GlcNAc,
M5: five mannose on core GlcNAc,
F(6)A1G(4)1: core fucosylated monoantennary with β1-4 linked Galactose,
A2[6]G(4)1: biantennary with β1-4 linked Galactose attached to α1-6 linked core mannose,
F(6)A2[6]G(4)1: core fucosylated biantennary with β1-4 linked Galactose attached to α1-6 linked core mannose,
F(6)A2[3]G(4)1: core fucosylated biantennary with β1-4 linked Galactose attached to α1-3 linked core mannose,
F(6)A2[6]G(4)1Ga1: fucosylated bianntennary with a β1,4-linked galactose directly attached to the α1,6-linked core mannose, and an α1,3-linked galactose attached to the β1,4-linked galactose,
F(6)A2G(4)2: equal to G2F,

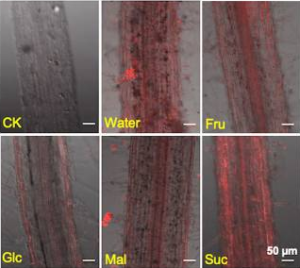 A red fluorescence-labeled Batillus subtilis strain was used with different sugars (Fru: fructose, Glc: glucose, Mal: maltose, and Suc: sucrose)
A red fluorescence-labeled Batillus subtilis strain was used with different sugars (Fru: fructose, Glc: glucose, Mal: maltose, and Suc: sucrose)