α2,6-Sialylation is upregulated in Severe COVID-19
A group from Department of Chemistry, University of Alberta, Edmonton, Alberta, Canada, etc. has reported that α2,6-Sialylation is upregulated in severe COVID-19.
https://pubmed.ncbi.nlm.nih.gov/35702159/
In influenza, severity of disease was found to be associated with levels of high mannose and the innate immune lectin MBL2. In SARS-CoV-2 infection, antibody glycosylation has been studied as a marker of severity. Antibodies to the spike protein were altered in severe patients, with lower fucosylation and sialylation observed. This has potential consequences for effector function. However, such studies have focused on a single protein type (IgG). To date there has been no work on the systemic glycomic response to SARS-CoV-2 infection in plasma and no analysis of infected tissues.
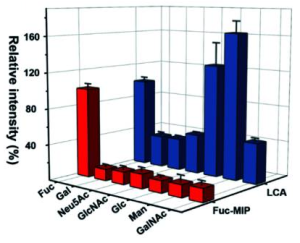
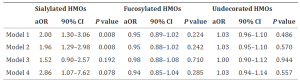
Herein, high-throughput analysis of plasma and autopsy sample glycosylation was performed from COVID-19 patients using a lectin microarray technology. It was revealed that plasma α2,6-sialic acid could be a marker of severity. This modification is known to increase the half-life of select proteins, including IgG. In plasma, it was found that the fraction of α2,6-sialylated C5 and C9 in severe COVID-19 patients is significantly upregulated. In line with this, it was observed that the staining for complement proteins C5 and C9 in COVID-19 autopsy samples got stronger.
However, the functional significance of sialylation on complement proteins remains poorly understood. Glycosylation can also play a role in controlling both serum half-life and resistance to proteolytic cleavage, which is of particular importance to this cascade. The α2,6-sialylation may be increasing half-life, prolonging the cell-mediated damage from the cascade. There may also be other effects of α2,6-sialylation on complement biology that have yet to be discovered. It will be needed to understand the functional impact of α2,6 sialylation and other glycans on complement.