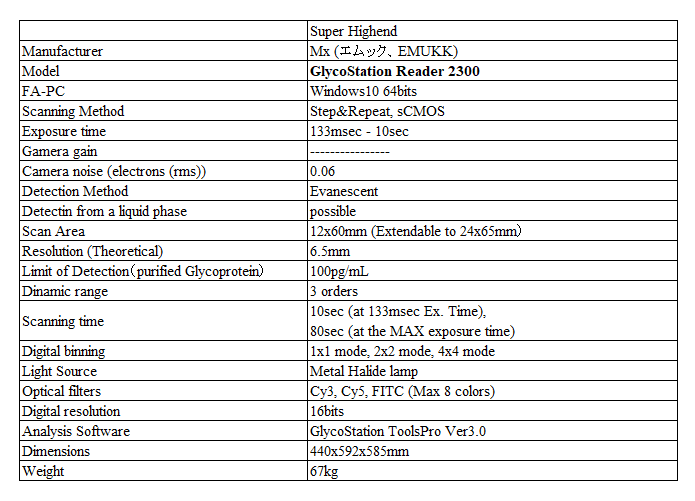
Spec. of GSR2300
The Spec. of GlycoStation® Reader 2300 (GSR2300) is summarized below.

High Sensitivity & Low Noise
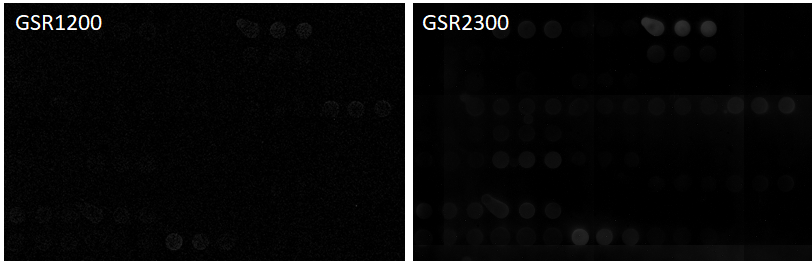
Compared to the first generation glycan profiler, GlycoStation® Reader 1200 (GSR1200), the major difference of the latest glycan profiler, GSR2300, is its low noise performance and high speed scanning. This is due to the difference in detection devices and innovation of the optical design. The GSR1200 used EMCCD, while the GSR2300 uses sCMOS. As shown below, the low noise characteristics of the GSR2300 are evident for samples with very low signal intensity. The figure below shows fluorescence images of a sample with very weak signal intensity acquired under the maximum sensitivity condition of each model. If you look closely at the images, you’ll notice that some spots appear faint. Additionally, you may notice that there are more faint spots in the image of GSR2300 than GSR1200.
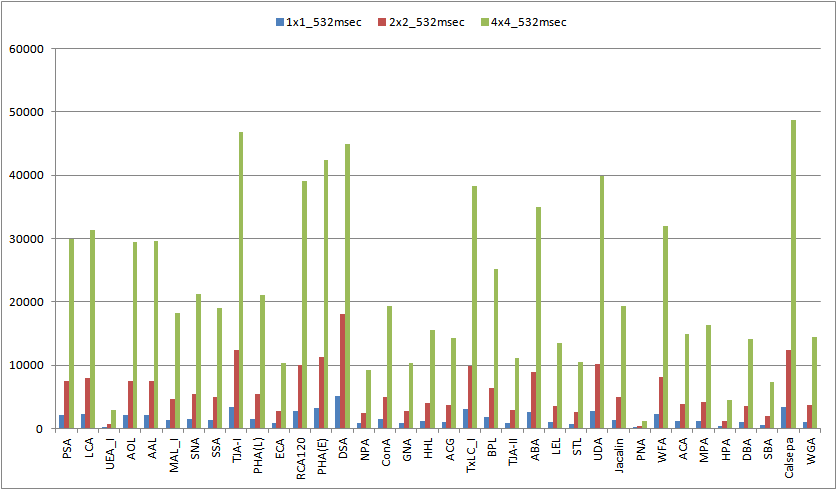
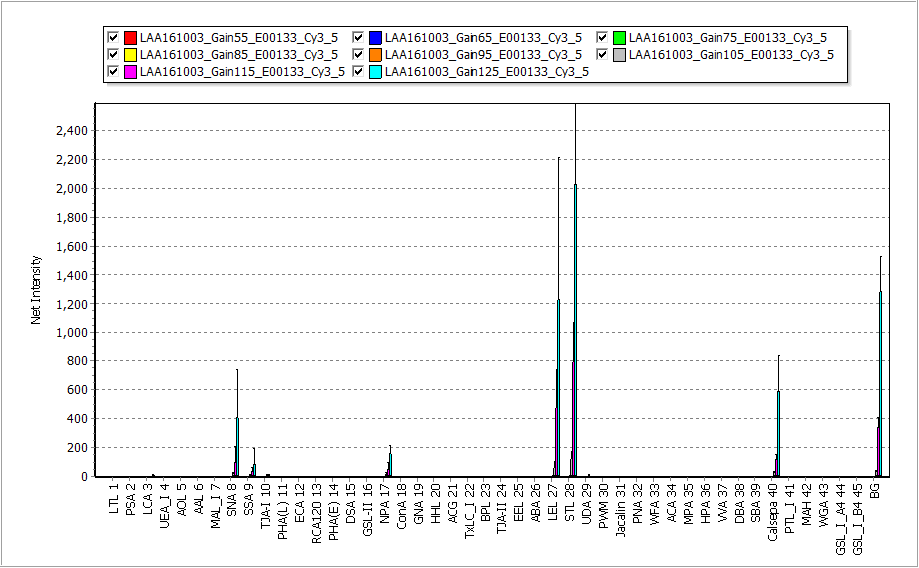
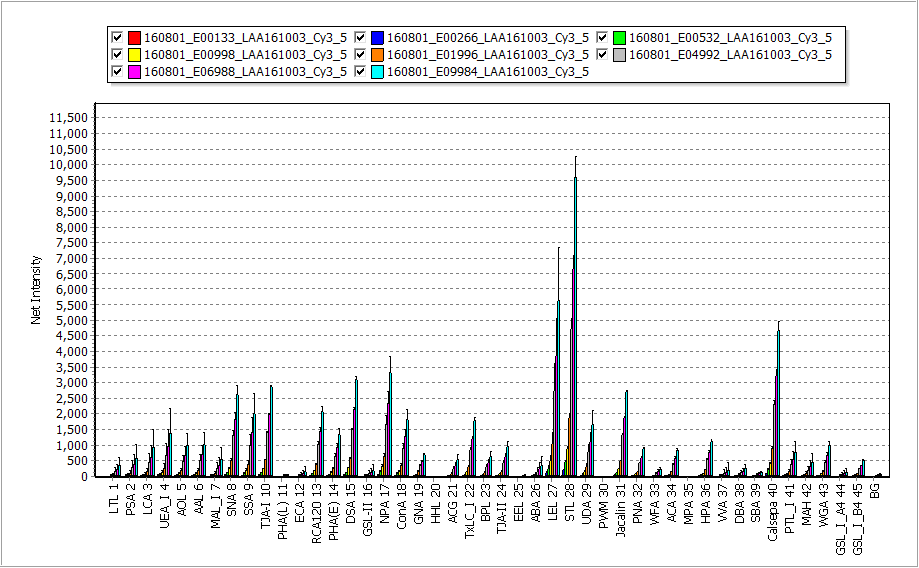
This subtle difference becomes clear when the brightness of the spot is digitized. The figure below is a graph of Net Intensity (i.e., Raw intensity minus Background) digitized with GlycoStation® ToolsPro. In the figure below, the upper figure shows the Net intensity graphed by digitizing the fluorescence image of GSR1200, and the lower figure shows the Net intensity graphed by digitizing it of GSR2300. It is obvious that GSR2300 clearly shows more lectins than GSR1200. This difference comes from the intensity of the BG (Background) signal shown on the right edge of each figure. In the case of GSR1200, the BG signal exceeds 1200, but in the case of GSR2300, the BG signal is below 100. The net Intensity is the result of subtracting this BG from the Raw Intensity, therefore in the case of the GSR1200, since the BG is too high, when the BG is subtracted from the Raw Intensity, all of the lectins with weak signal intensities result in negative values. However, in the case of GSR2300, since the BG is very weak, even lectins with such weak signal intensities can remain as positive values. This example clearly demonstrates the low noise characteristics of GSR2300. As a result, the linearity in the low-signal region is improved resulting in the overall improvement in the dynamic range.
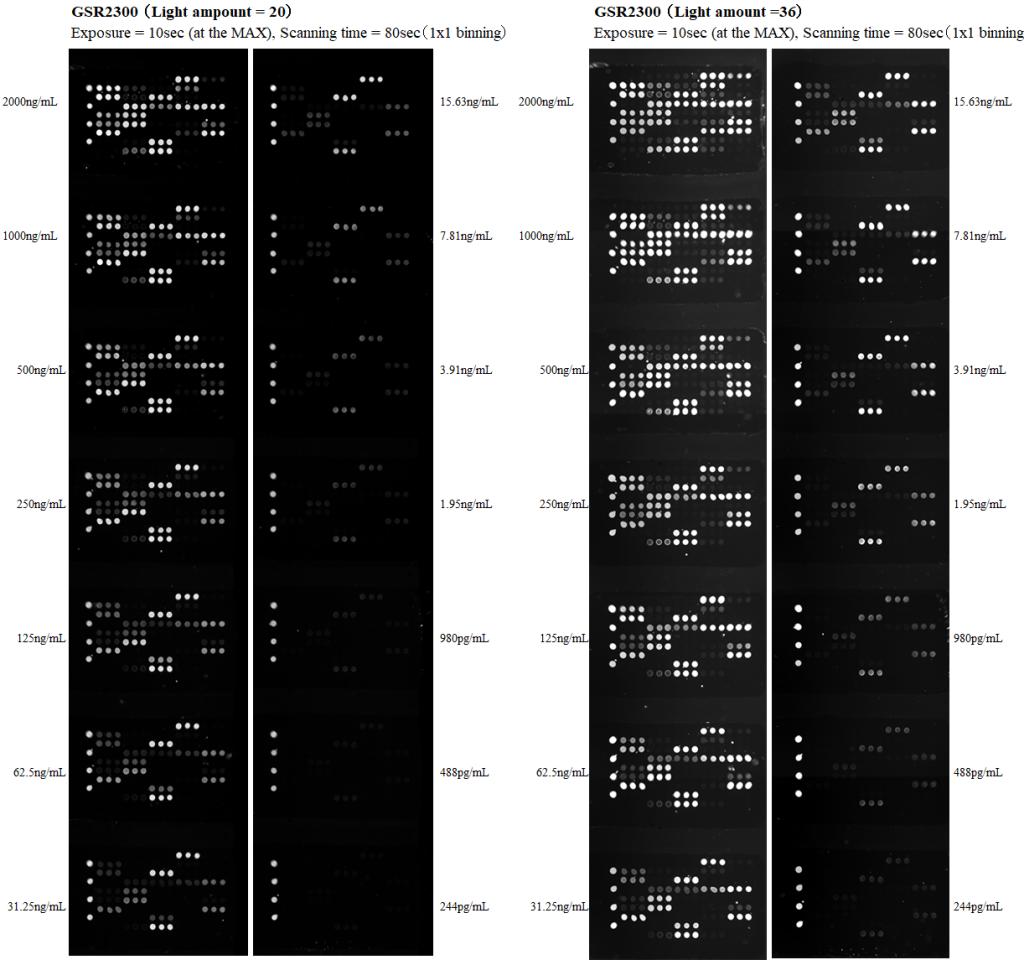
Next, we show the high sensitivity characteristics of GSR2300. GSR2300 uses a metal halide lamp as the excitation light source. The excitation light intensity is adjusted in 8 bits (255 gradations), and as a standard excitation light intensity, we recommend a light intensity setting value between 20 and 40 (the maximum value = 255). By increasing the excitation light intensity, the sensitivity naturally increases, but at the same time, the BG also increases, so that this range is the recommended setting value from a viewpoint of S/N ratio. However, if you do not care lectins with strong signal intensities can be saturated under higher sensitivity conditions, it is possible to increase the excitation light intensity further to detect weak signals. The figures below shows fluorescence images when the excitation light intensity setting is increased from 20 to 36. By changing the excitation light intensity, the sensitivity can be adjusted in conjunction with adjusting the exposure time of the sCMOS. The LOD (Limit of Detection) value, of course, varies depending on the sample, but for antibody drugs, it is around 100 pg/mL.
High Speed Scanning
In GSR2300, fluorescence images of the lectin microarrays are acquired by 7 step & repeat scans in the y-axis direction (longitudinal direction of the slide glass). Since the maximum exposure time of sCMOS is 10 seconds, the scan time at the high sensitivity condition is 80 seconds, including the stage movement time. The shortest exposure time of this model is 133msec, in this case, the scan time is only 10 seconds.
The installed sCMOS has a function called Digital binning. Digital binning is a method of handling several adjacent cells together as a single cell by software. Sensitivity depends on the total amount of light that physically enters the cell, so the sensitivity increases linearly as the number of cells integrated as a single cell increases. However, since merging cells with digital binning reduces the optical resolution, there is a limit to the number of cells that can be merged from a practical point of view.
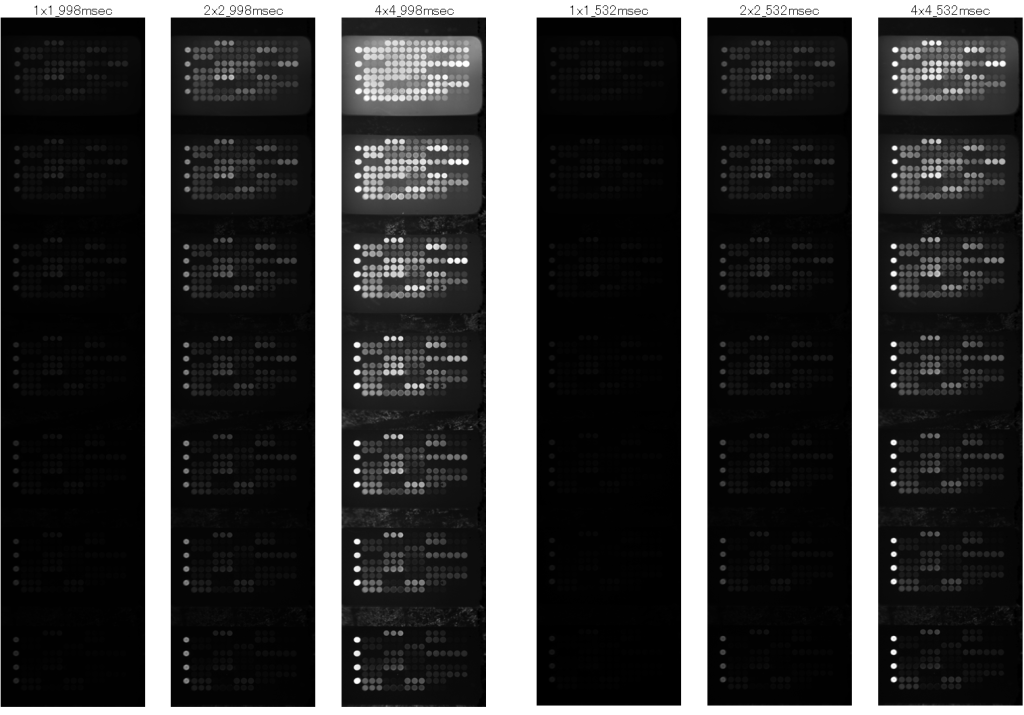
GSR2300 is equipped with a 1×1 mode for the normal mode, a 2×2 mode that integrates two cells vertically and horizontally (i.e. total 4 cells), and a 4×4 mode that integrates four cells vertically and horizontally (i.e. total 16 cells). The increase in sensitivity due to digital binning is drastic and is shown in the figures below. The left figure shows the case of exposure time = 998 msec, and the right figure shows the case of exposure time = 532 msec. In both figures, from the left side, the normal 1×1 mode, 2×2 mode, and 4×4 mode with digital binning are shown. A linear increase in sensitivity can be seen. The bottom figure shows the digitization of this fluorescence image. The sensitization effect is clearly shown.
By using the digital binning function, it is possible to shorten the scanning time to 15 seconds while keeping the highest sensitivity condition of GSR2300, successfully achieving both ultra-high sensitivity and ultra-high speed scanning.