Keyman speaks
Five researchers talk about the application of comparative glycan profiling analysis using a lectin microarray/evanescent field fluorescence excitation technology from their respective research fields. Dr. Hirabayashi summarized the overall picture from a view point of paradigm shift in glycan analysis technology, Dr. Zhang introduced Lectin micoarray as an effective tool for assessing glycosylation of therapeutic proteins, Dr. Onishi focused on the development of prognostic markers for IgA nephropathy from a single drop of urine. Dr. Matsuda focused on the development of biomarkers for liver fibrisis, and Dr. Shimoda mentioned about assessment of glycosylation of extracellular vesicles as biomarkers and for drug delivery applications.

Development of Urinary Diagnostic Biomarker for IgA Nephropathy by Lectin Microarray
Yasuhiro Onishi, et. al., Okayama University School of Medicine (2022)
N-glycan structures of Wisteria floribunda agglutinin-positive Mac2 binding protein in the serum of patients with liver fibrosis by Lectin Microarray
Atsushi Matsuda et.al., Keio University School of Medicine (2021)
Assessment of Surface Glycan Diversity on Extracellular Vesicles by Lectin Microarray and Glycoengineering Strategies for Drug Delivery Applications
Asako Shimoda, et. al., Graduate School of Engineering, Kyoto University (2021)
The use of lectin microarray for assessing glycosylation of therapeutic proteins
Lei Zhang, et. al., FDA (2016)
Development of lectin microarrays, an advanced system for glycan profiling analysis
Jun Hirabayashi, Research Center for Stem Cell Engineering, AIST (2013)
Overview of Glycan Profiling Technology: GlycoStation®

The central dogma of life is that “proteins are made from genes”, and proteins translated from genes are modified by other molecules (glycans: carbohydrates) in their final stages to complete their forms. The glycan itself is not encoded by the gene, but a protein called glycosyltransferase that modifies a protein with glycan is encoded by the gene. For those who are unfamiliar with glycans, sugar may come to mind when talking about glycan. Sugar is a type of disaccharide made of glucose and fructose, and is distinguished from glycans. Glycans are by no means sweet. They bind to amino acids (asparagine, serine, and threonine) at specific sites in proteins and have a complex branching structure like a tree. The structure of the binding site is universal as its core, but as the glycan extends, the side chain grows like a branch of a tree, and “leaves of various shapes: monosaccharides” are attached to it. In humans, there are 10 types of monosaccharides (galactose, mannose, N-acetylglucosamine, N-acetylgalactosamine, and sialic acid, and so forth).
The functional molecules in life are fundamentally proteins, and the functions of proteins are greatly affected by the glycan modification. There are two types of proteins: secreted types and non-secreted types that stay inside cell and/or on the cell membrane. Most proteins expressed on the cell membrane function as receptors and are involved in signal transmission from the outside into the cell. Since most of these receptors are modified with glycans, the glycans are present on the outermost surface of the cell when viewed from the outside. In other words, glycans are at the forefront of intercellular signal transduction and serve as the “face of the cell.”
Let me explain some typical examples, to make the image of glycans clearer. For example, depending on whether or not an antibody drug (IgG Type I) is modified with core fucose, the activity of immune cells will change significantly, and the efficacy changes drastically by 100 times. The new coronavirus (SARS-CoV-2) transforms itself into a stealth fighter by wearing glycans like a cloak, bypassing the human immune system and binding to a receptor called ACE2 in the respiratory tract to initiate infection. In cancer, when the malignancy increases, the branches of glycan are trimmed off increasing the invasive capacity of cancer. When proteins in the blood become old, sialic acid is removed and galactose appears on the top, and thereby old proteins are selectively destroyed in the liver and excreted. In this way, glycans are deeply involved in the regulation of protein functions everywhere.
There is a group of proteins that specifically recognize such glycans, and these glycan binding proteins (excluding enzymes and antibodies) are called lectins. Humans are said to have about 200 lectins. It is actually lectins that give meanings to these “existing” glycans.
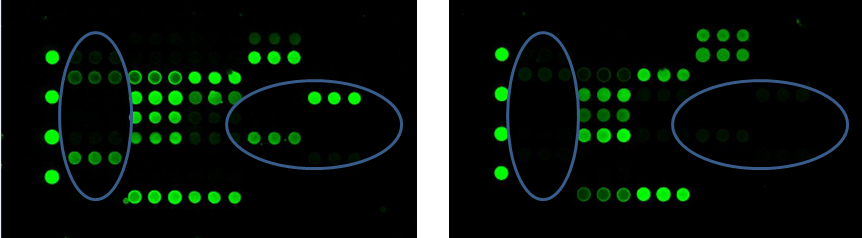
GlycoStation® is the technology for glycan profiling using a lectin microarray, which is a slide glass array of such lectins immobilized. By combining lectins with different glycan binding specificities, the characteristics of glycan structures can be profiled. As the term profiling suggests, this technology is not a technology for identifying complex glycan structures completely. However, depending on the usage and application, it is not necessary to completely identify the glycan structures. Why? that is because the molecules that recognize the characteristics of glycans existing in the body and activate the related signal path are lectins. Glycan profiling technology using lectin microarrays can be said to be a technology that makes it possible to easily identify “structure data of glycans that are meaningful for life”. From a view point of analytical chemistry, a technology that cannot completely identify the structure of glycans may feel inaccurate and uncomfortable, but there are many applications where it is sufficient if the necessary information for life can be obtained. For example, biomarker discovery and screening in the development process of therapeutic proteins are the most suitable applications. By using this technology, changes in glycosylation of cells and proteins can be easily identified by lectins. You can easily get the information that life really needs and uses, such as the amount of sialic acid increased, the amount of core fucose also increased, and so forth. This advantage enhances the effectiveness of glycan profiling technology using lectin microarrays greatly in several applications as mentioned above.
It was in 2005 that lectin microarrays appeared. The pioneering microarray in the biotechnology field is, as you know, the DNA microarray. Microarray technology is always paired with a scanner that reads it. There are many microarray scanners in the world. GlycoStation® is greatly differentiated from these existing scanners by its detection method. It is no exaggeration to say that Mx’s GlycoStation® is the technology that is able to extract the most accurate information in the world from scanning of lectin microarrays and glycan arrays. This is because GlycoStation® is a technology that enables non-destructive detection of very weak intermolecular interactions. The interaction between glycans and lectins is far weaker than the interaction that forms the double helix of DNA or the antigen-antibody reaction. GlycoStation® uses an evanescent field (near field) for the detection of such weak intermolecular interactions. Devices that use near fields have existed for a long time, including confocal microscopes, but the GlycoStation® Reader 1200 was the first one that generates evanescent field over the entire surface of a slide glass and can detect intermolecular interactions over such a large area. By using evanescent field, it is possible to detect the interaction between lectins and glycans non-destructively from a liquid phase. The current scanner is GlycoStation® Reader 2300 (GSR2300), and the low cost version is GlycoSuperLite 2200 (GSL2200). We do not call the GSR2300 simply a scanner, but call it “glycan profiler” to embody the above meaning.