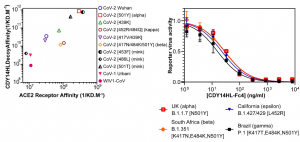
A single vaccination is sufficient to boost immunity to high levels among previously infected individuals
A group from Northwestern University Feinberg School of Medicine, USA, etc. has reported on neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8276631/
The following three groups were compared:
(1)COVID-19 group: previously recovered from a confirmed outpatient case of COVID-19,
(2)Seropositive group: seropositive for anti-SARS-CoV-2 receptor binding domain (RBD) IgG but no acute virus diagnostic test for COVID-19,
(3)Seronegative group: seronegative for prior SARS-CoV-2 infection prior to vaccination.
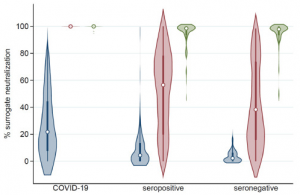
The COVID-19 group had median% neutralization (22.2 vs. 4.4, p < 0.001) in comparison with the seropositive group. The COVID-19 group had significantly higher median% neutralization (99.9 vs. 56.5, p < 0•001) in comparison with the seropositive group. Post-dose 1 responses were higher for the seropositive group in comparison with the seronegative group, but %neutralization (56.5 vs. 38.2, p = 0•12) were not significantly different. The COVID-19 group had higher post-dose 2 median% neutralization (99.9 vs. 98.5, p<0•001) in comparison with the seropositive group. The seropositive and seronegative groups did not differ significantly in post-dose 2 median% neutralization (98.5 vs. 97.9, p = 0.46). As a result, a single dose is sufficient to boost immunity to high levels among previously infected individuals.