A group from University of Nebraska Medical Centergrid.266813.8, Omaha, Nebraska, USA, etc. has reported on adhesion mechanism of Staphylococcus epidermidis to corneocytes.
https://journals.asm.org/doi/10.1128/mBio.02908-20
Staphylococcus aureus (S. aureus) is the most significant cause worldwide of skin and skin structure infections. S. aureus is infrequently isolated from the skin from healthy subjects. This suggests that Staphylococcus epidermidis (S. epidermidis) and other bacteria colonizing the skin are highly effective at inhibiting colonization of this pathogen.
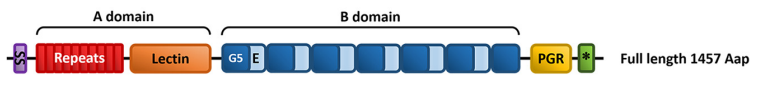
It was found that S. epidermidis expresses a full-length accumulation-associated protein (Aap) on its cell membrane, and the Aap adheres to glycans expressed on corneocytes. Aap is composed of two distinct A and B domains. The A domain is comprised of a repeat region and a conserved L-type lectin domain. A domain-mediated binding is dependent upon the lectin subdomain but requires the A-repeat domain and the B domain for maximum ligand-receptor interaction.
The glycan binding specificity of Aap was investigated by changing glycan structures with glycosidases. As a result, it was found that Neu5Ac, fucose, and galactose contribute binding to Aap, either individually or together, but no affinity to GlcNAc and mannose.
Since S. aureus contains an Aap orthologue, the common mechanism for corneocyte adherence will be also applied in this strain. Therefore, it will be likely that S. epidermidis has the ability to outcompete S. aureus, potentially via adherence to carbohydrate molecules decorating corneocytes.