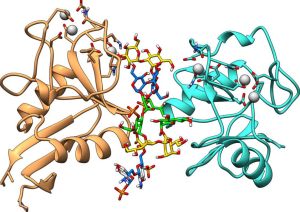
DC-SIGN recognizes the outer core oligosaccharide of LPS expressed on Gram-negative bacteria
Department of Chemical Science, University of Naples Federico II Via Cinthia 4, Naples, Italy, etc. has reported about molecular recognition of LPS by DC-SIGN.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10828809/
Lipopolysaccharides (LPS) are peculiar glycolipids which represent the major components of the external leaflet of the gram-negative bacteria outer membrane. They consist of three structurally and genetically distinct domains: the lipid A, integrated in the outer membrane; the core oligosaccharide (OS), in turn composed of inner and outer core regions; and the distal O-specific polysaccharide (O-PS) chain, that extends outwards the bacterial surface
Structurally speaking, it is a dodecasaccharide composed of two residues of galactose and three glucose units in the outer core region and three L-glycero-D-manno-heptoses and two 3-deoxy-D-manno-oct-2-ulosonic acids (Kdo), in the inner core portion; the two glucosamine residues at reducing end belong to the lipid A moiety.
One of the main representatives of transmembrane C-type lectins is DC-SIGN also known as CD209. This lectin is found on macrophages, monocytes, and is mainly expressed by dendritic cells which act as potent phagocytic cells, and it is know that DC-SIGN belongs to the mannose receptor family. On the other hand, it has been shown that the DC-SIGN induced phagocytosis of E. coli occurs in the absence of O-antigen polysaccharides, and in the presence of a complete core OS.
In this study, it was found that DC-SIGN binds to the outer core pentasaccharide (composed of two residues of galactose and three glucose units), which acts as a crosslinker between two different tetrameric units of DC-SIGN.