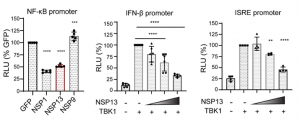
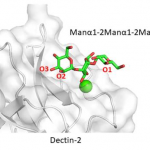
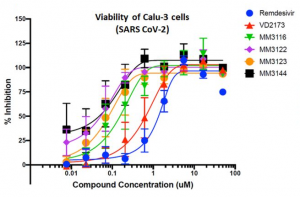
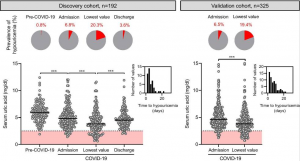
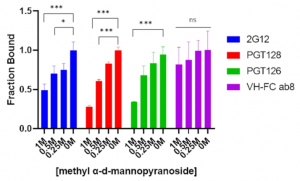
SARS-CoV-2 viral nonstructural proteins NSP1 and NSP13 can block interferon activation, leading to escaping from innate immune attacks
A group from University of Pennsylvania Perelman School of Medicine, USA, etc. has reported that SARS-CoV-2 viral nonstructural proteins NSP1 and NSP13 can block interferon activation via distinct mechanisms.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0253089
The innate immune antiviral activity provides the first barrier of protection against viral pathogens. Upon RNA virus infection, viral pathogen associated molecular patterns such as viral RNA, spike protein are sensed by cytosolic pattern recognition receptors including RIG-I, MDA5, and TLR family members. RIG-I-viral RNA binding initiates a downstream signaling cascade through a central innate immune adaptor protein, Mitochondrial antiviral-signaling protein (MAVS). Once MAVS is activated, the kinases Tank-binding kinase1 (TBK1) and I-kappa-B epsilon kinase (IKKε) phosphorylate Interferon Regulatory Factor 3 (IRF3), and leads to its nuclear translocation and transcriptional induction of interferon-beta (IFN-β). Secreted IFN-β binds to the IFN-ɑ/β receptors (IFNAR) on neighboring cells, and initiates the interferon response and signaling through the JAK/STAT receptor kinases to produce interferon-stimulated genes (ISGs).
It was shown that SARS-CoV-2 viral nonstructural proteins NSP1 and NSP13 limit IFN response promoter and NF-κB activation, especially NSP1 inhibits nascent host translation and NSP13 interacts with TBK1 and blocks TBK1-mediated IFN activation.