A group from Tohoku Medical and Pharmaceutical University has reviewed 11 kinds of mammalian lectins responsible for pathogen recognition.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8185196/
From this review, three C-type lectins were highlighted here for your information.
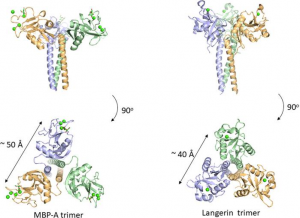
Many C-type lectins show selectivity toward mannose residues and this mannose binding is utilized for microbe sensing. One of the most studied examples is mannose-binding protein, MBP, which is known to activate the complement lectin pathway. Why does mannose recognition play this role in detecting non-self when mannose residues occur frequently in mammalian N-glycans, e.g., high-mannose glycan. The likely explanation is in the higher spatial density of mannose residues on bacteria relative to that of mammalian glycans. The C-type lectin domain has a Ca2+ ion coordinated with the OH3 and OH4 of the mannose. The affinity of 1:1 binding is weak with a dissociation constant of roughly 1 mM, however, the presentation of trimeric binding sites in MBP domains could interact with the multiple terminal mannose residues presented on microbes. The spacing between the mannose binding sites is around 50 Å, and is eminently suitable for binding packed terminal mannose residues with high affinity, but not single endogenous high mannose glycans. Langerin is also a C-type lectin with a coiled-coil region and a neck region in a trimeric structure, and has a distance of roughly 40 Å between binding sites. A trimeric oligosaccharide ligand with appropriate linker length for the 40 Å distance between each binding site has been reported for Langerin, with 1,000-fold higher affinity over the monomeric ligand.
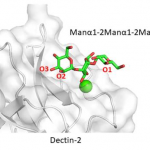
As for Dectin-2, the binding site accommodates internally positioned Manα1-2Man of mannans and other polysaccharides, whereas other C-type lectins like DC-SIGN and langerin bind only terminal Manα1-2Man structures. Recognition of internal mannose residue is advantageous in that multiple binding sites are presented toward lectin receptor. Dectin-2 is thus suitable for binding to longer mannan polysaccharides.