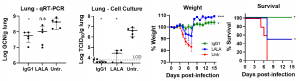
A group from Biological Defense Program, DSO National Laboratories, Singapore, etc. has reported on Fc-mediated effector functions of a potent SARS-CoV-2 neutralizing antibody.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0253487
Antibody-Dependent Enhancement (ADE) of disease remains a major concern for the use of anti-SARS-CoV-2 antibodies as therapeutics. ADE can occur if Fcγ Receptor (FcγR) engagement mediates an increase in the infection of phagocytic cells. Due to the potential of ADE, several ongoing SARS-CoV-2 antibody programs have chosen to use Fc isotypes that do not engage FcγR, like the IgG4 isotype, and engineered variants such as those carrying FcγR-null LALA variant. However, these may be counterproductive because the signaling mechanisms underpinning the efficacies of these antibodies, particularly the ability of FcγR engagement to induce other antiviral responses such as ADCC will be killed. To address these questions, authors isolated and characterized a RBD-binding neutralizing IgG1 antibody, named SC31, from an early convalescent patient. Authors assessed the impact of Fc functionality on its therapeutic efficacy by comparing SC31 with its LALA variant and demonstrated that the engagement of Fc receptors by SC31 triggers additional IFN-γ-mediated antiviral responses but importantly do not induce ADE.
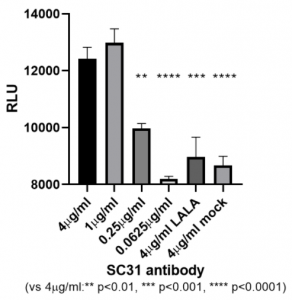
To determine the role of Fc-mediated effector functions in the therapeutic efficacy of SC31, the abilities of SC31 and its LALA variant were compared. The upstream activation of the FcγRIIIa ADCC signalling pathway was evaluated using a Jurkat reporter cell line expressing FcγRIIIa and with ADCC reporter assay after co-culture with target HEK293 cells expressing membrane-bound SARS-CoV-2 Spike protein. In contrast to its LALA variant, SC31 was confirmed to induce a dose-dependent activation of ADCC signaling.