A group from National Centre for Biological Sciences (TIFR), Bengaluru, India, etc. has reported on SARS-CoV-2 infection mechanisms based on endocytosis.
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009706
A key step in successful virus infection is the release of viral genomic content into the host cell cytoplasm. To achieve this, viruses bind to specific cell surface receptors and subsequently undergo membrane fusion either directly at the plasma membrane or following endocytic uptake. Both alternatives of entry are feasible for SARS-CoV-2 infections depending on the availability of receptors and proteases at the host cell surface. Although angiotensin converting enzyme 2 (ACE2) is a well-studied receptor for SARS-CoV-2, other receptors and co-receptors have been discovered from a number of groups. Additionally, SARS-CoV-2 requires proteolytic processing of the viral envelope Spike protein by host cell proteases to gain entry. Therefore, these viruses can directly fuse at the cell surface if the Spike protein is cleaved by a cell surface serine protease like TMPRSS2, or utilize an endo-lysosomal route for fusion, where the Spike protein is primed by cysteine protease cathepsins. So, viral entry and infection in different host cells is dependent on the expression of these key host factors (receptors such as ACE2 and protease like furin, TMPRSS2, and cathepsin.
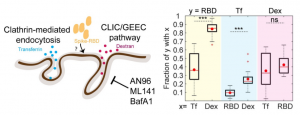
The CLIC/GEEC (CG) pathway is a clathrin-independent endocytic pathway mediated by uncoated tubulovesicular primary carriers called clathrin-independent carriers (CLICs) which arise directly from the plasma membrane and later mature into tubular early endocytic compartments called Glycosylphosphotidylinositol- anchored protein (GPI-AP) enriched compartments (GEECs).
Authors studied the endocytosis of receptor binding domain (RBD) of SARS-CoV-2 Spike protein in gastric epithelial cells (AGS) in the presence and absence of ACE2. AGS can be considered as a cell line with undetectable levels of endogenous ACE2. It was shown that RBD is endocytosed via the CG endocytic pathway (rather than clathrin-mediated endocytosis (CME) pathway) and its uptake is sensitive to pharmacological perturbations of this pathway in AGS cells.
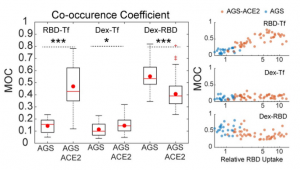
To determine the effect of ACE2 on uptake of RBD in AGS cells, a stable AGS cell line ectopically expressing ACE2 (AGS-ACE2) was generated; the expression of ACE2 was confirmed using qPCR and western blot analysis. RBD uptake in AGS-ACE2 was about 3-fold higher than AGS cells. On characterizing the RBD endocytic itinerary in AGS-ACE2 cells, an increase in the co-occurrence of RBD with transferrin was observed, and slightly reduced co-occurrence of RBD with dextran compared to AGS cells was also observed. This indicates that in addition to trafficking via the CG pathway, RBD is now trafficked via the CME in AGS-ACE2 cells.
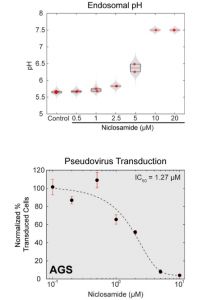
Finally, it was shown that niclosamide neutralizes endosomal pH and inhibits SARS-CoV-2 infection as follows.