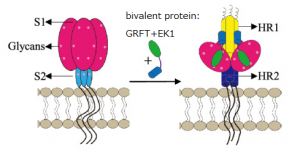
A group from Shanghai Institute of Infectious Disease and Biosecurity, Fudan University, Shanghai, China, has developed a bivalent protein targeting oligo-mannose in SARS-CoV-2 Spike and HR1 domain in S2 subunit to inhibit SARS-CoV-2 infection.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8264481/
To enter target cells, SARS-CoV-2 binds to its receptor ACE2 on the host cell through the receptor-binding domain (RBD) in S1 subunit of spike (S) protein. Such binding triggers conformation changes in the S2 subunit of S protein, resulting in the formation of a six-helix bundle (6-HB) between the heptad repeat 1 and 2 (HR1 and HR2) domains, thus bringing viral and target cell membranes together for fusion. Therefore, both S1 and S2 subunits can serve as important targets for the development of SARS-CoV-2 fusion and entry inhibitors. The sequence of S2 subunit is more conserved than that of S1 subunit, making it a better target for developing broad-spectrum CoV entry inhibitors.
Authors aimed to design and construct a bivalent protein consisting of antiviral lectin GRFT and pan-CoV fusion inhibitory peptide EK1 and evaluate its inhibitory activity and mechanism of action against infection by SARS-CoV-2 and its mutants, as well as other human coronaviruses (HCoVs). Three types of recombinant plasmids encoding bivalent proteins (GRFT and EK1) with different linkers in the length, GRFT-L15-EK1 (GL15E), GRFT-L25-EK1 (GL25E), and GRFT-L35-EK1 (GL35E), containing linkers L15 (GGGGS)3, L25 (GGGGS)5, and L35 (GGGGS)7 between the GRFT and EK1 components.
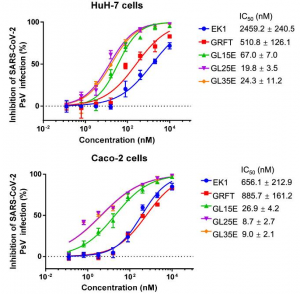
It was found that GL25E was the best to inhibit SARS-CoV-2 infection as shown below.