A group from Department of Gastroenterology of the Second Affiliated Hospital, School of Medicine and Life Sciences Institute, Zhejiang University, Hangzhou, China, etc. has reported that 35B5 potently neutralizes SARS-CoV-2 Omicron and other variants by causing significant conformational changes within a conserved N-glycan switch that controls the transition of RBD from the “down” state to the “up” state, which allows recognition of the host entry receptor ACE2.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8960183/
Neutralization of SARS-CoV-2 by antibodies is carried out through mechanisms including ACE2 competition, ACE2 molecular mimicry, and Fc-receptor-mediated neutralization. N-linked glycosylation has important roles in viral pathology, including mediating protein folding and stability and shaping viral tropism. Glycosylation shields specific epitopes to facilitate viral immune evasion. Beyond the shield function, the glycans at N165 and N234 from the N-terminal domain (NTD) in SARS-CoV-2 act a molecular switch to control the conformational transition of the RBD from the “down” state to the “up” state, which is required for the receptor to bind to ACE2. N165 and N234 are conserved in SARS-CoV-1 and MERS-CoV, highlighting the common mechanism of RBD conformational transition in S proteins.
The Omicron S protein has significant antigenic shifts and structural changes, leading to immune escape of the Omicron variant from most mAbs. Previously, it had been shown that 35B5 has neutralizing activities against the WT and the Beta and Delta variants of SARS-CoV-2. 35B5 dissociates the S trimer and neutralizes SARS-CoV-2. In this study, it was found that 35B5 could neutralize the Omicron variant with a potent neutralizing efficacy much higher than that of many other neutralizing antibodies.
The actual reason why the S protein dissociates is that 35B5 displaces the conserved glycan switch from the RBD, leading to the unstable up states of the RBD and eventually causing the shedding of S1 from the S trimer. The glycan-displacement action of 35B5 represents an unprecedented neutralizing action of mAbs against SARS-CoV-2, which is different from the previously determined ACE2 competition and molecular mimicry mechanisms utilized by class 1 and 2 RBD mAbs and the Fc-receptor-mediated neutralizing mechanisms of class 3 and 4 RBD mAbs
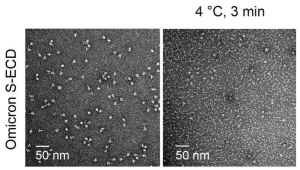 This figure shows that in vitro incubation with 35B5 led to complete dissociation of the Omicron S trimer.
This figure shows that in vitro incubation with 35B5 led to complete dissociation of the Omicron S trimer.
