Spatial and abundance mapping of plant N-glycosylation cellular heterogeneity inside Soybean Root Nodules
A group from Environmental Molecular Sciences Laboratory, Earth and Biological Sciences Directorate, Pacific Northwest National Laboratory, Richland, WA, USA, etc. has reported about spatial and abundance mapping of plant N-glycosylation cellular heterogeneity inside Soybean Root Nodules.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9150855/
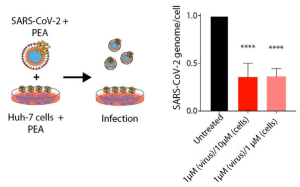
Although glycans are significant mediators of the nodulation process and plant-microbe interactions, there are no studies on whether protein N-glycosylation is affected by nitrogen fixation. Therefore, we compared N-glycome spatial and abundance profiles in soybean nodules infected with wild-type rhizobia and infected with nifH-mutant rhizobia incapable of efficiently fixing atmospheric nitrogen. The nifH-mutant still infects the root and forms the nodules, but cannot fix the nitrogen.
It was found that the majority of N-glycans showed an overall higher abundance in the nifH-mutant nodules, only a few N-glycans showed non-significant changes between WT and nifH-nodules, while no N-glycan was found significantly more abundant in WT. Notably, all glycans with LewisaN-がた-epitope showed significantly higher abundance in the nifH-mutant nodule tissue. In contrast, the level of truncated N-glycans was conserved between the two types of nodules.
Proteomic results identified nine glycoproteins as potentially major carriers of Lewisa glycans as possible candidates for further characterization.
The nine proteins identified were as follows; phytocyanin domain-containing protein (I1LWP0), amidohydro-rel domain-containing protein (I1L921), dirigent protein (I1JL51), peroxidase (I1MP39), germin-like protein (C7S8D5), an uncharacterized copper ion binding protein with oxidoreductase activity (I1MUX7), an uncharacterized enzyme with mannosyl-oligosaccharide glucosidase activity (I1K3K7), and two uncharacterized proteins with unknown molecular function (I1K380, and C5HU39).
Glycoproteins with those specific glycans may be involved in mediating efficient biological nitrogen fixation or downstream effects during bacteroid infection. Indeed, all these nine proteins have known or predicted functions related to biological nitrogen fixation or root development, and it is plausible that their Lewisa-type glycosylation may be involved in their function.