A group from Faculty of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo, Kashiwa, Chiba, Japan, etc. has reported a novel method to detect O-GlcNAcylated proteins specifically and with high sensitivity.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9126400/
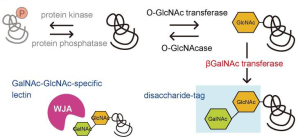
O-GlcNAc modification of serine or threonine occurs only on nuclear or cytoplasmic proteins. This modification is significantly different from N-glycosylation or O-glycosylation from several perspectives.
(1) O-GlcNAcylation occurs in the nucleus or cytoplasm of the cell, whereas general glycosylation occurs in the luminal regions of the endoplasmic reticulum and Golgi apparatus.
(2) O-GlcNAcylation is a reversible reaction via the actions of O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), although N- and O-glycosylation are irreversible.
(3) general N- and O-glycans on proteins are sequentially processed and elongated with many types of glycosyltransferases. However, O-GlcNAc residues cannot be modified by other monosaccharides.
In this study, to detect O-GlcNAc modification of proteins, especially in the nucleus of the cell, expression vectors encoding soluble β4GalNAc-TA were constructed with a nuclear localization signal (NLS) from the SV40 large T antigen at the N- or C-terminus, respectively, and let β4GalNAc transferase transfer GalNAc to the GlcNAc residue of the O-GlcNAcylated proteins forming GalNAcβ1-4GlcNAc disaccharide. Then, Wisteria japonica agglutinin (WJA) was used as a probe to detect GalNAcβ1-4GlcNAc disaccharide, because WJA is highly specific for GalNAcβ1-4GlcNAc.
This method was named as disaccharide-tag method.
The binding between GalNAc-GlcNAc used in this method and WJA lectin has a stronger Ka of 1.4 x 105 M-1 which enables higher sensitivity than any other methods. In addition, it should be emphasized that sugar chains with the GalNAc-GlcNAc sequence were hardly expressed in animal cells. The GalNAc-GlcNAc structure is found at the non-reducing end of the N-type sugar chain of pituitary glycoprotein hormones (leutropin, thyrotropin, follitropin), which serves as a clearance signal from the blood stream. These glycoprotein hormones are present only in the pituitary gland and are not expressed in other tissues and cells.