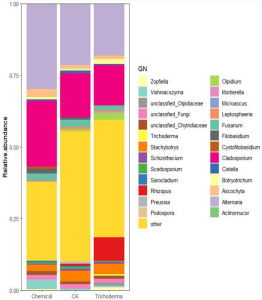
Innate immune lectin galectin-7 (Gal-7) binds a variety of distinct microbes and kills
A group from Joint Program in Transfusion Medicine, Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, 77 Avenue Louis Pasteur, Boston, MA 02115, USA, etc. has reported that the innate immune lectin galectin-7 (Gal-7) binds a variety of distinct microbes, all of which share features of blood group-like antigens.
<a href=”https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9218387/”>https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9218387/</a>
It has been known that several members of the galectin family, including galectin-3 (Gal-3), galectin-4 (Gal-4), and galectin-8 (Gal-8), possess the ability to bind blood group A and B with high affinity. Although prior studies demonstrated that Gal-3, Gal-4, and Gal-8 are capable of binding and killing microbes that utilize blood group-like molecular mimicry, the extent to which other members of the galectin family likewise share the ability to provide innate immunity against molecular mimicry remains incompletely defined.
In this report, it was demonstrated that Gal-7 also possesses the ability to bind and kill microbes that utilize blood group molecular mimicry. However, unlike Gal-3, Gal-4, and Gal-8, Gal-7 exhibits very little affinity for mammalian A and B antigens. Despite this, Gal-7 exhibited high specificity toward multiple microbes that express glycans with blood group-like features. These results demonstrate that antimicrobial activity among galectin family members is not limited to Gal-3, Gal-4, and Gal-8, but that Gal-7 likewise possesses the ability to bind and kill microbes expressing blood group-like antigens.
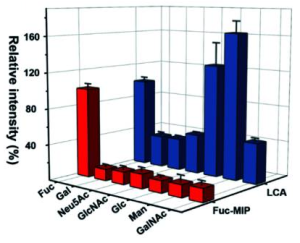
From microarray studies using CFG glycan arrays, at 0.04 μM, Gal-7 engaged only three glycan structures out of nearly 600, a <strong>type 2 (Galβ1-4GlcNAc) blood group A</strong> structure, and two <strong>polylactosamine (polyLacNAc, i blood group)</strong> containing structures capped with the H antigen. At 3.3 μM Gal-7 exhibited binding toward additional glycans, including several glycans containing the blood group B antigen. At 10 μM, Gal-7 is bound to a common non-blood group containing glycans recognized by other galectin family members, including biantennary N-glycans and those with poly-N-acetyllactosamine (polyLacNAc) structures. The selective binding specificity of Gal-7 over a broad range of concentrations distinguishes it from prior reports on other galectins and suggests that unlike Gal-3, Gal-4, and Gal-8, Gal-7 may not readily engage blood group positive microbes.
Although the mechanism whereby Gal-7 and other galectins kill microbes is unclear, the results of this study suggest that an ancient carbohydrate binding protein family expressed in mammals exists that can target a variety of microbes, each of which utilize features of blood-group molecular mimicry.