A group from Department of Chemistry, University of Wisconsin-Madison, Madison, WI 53706, USA has reported existence of O-glycosylation unique to SARS-CoV-2 Omicron variant.
https://www.biorxiv.org/content/10.1101/2022.02.09.479776v2.full
HEK293 expressed S-RBD protein arising from WT (WA1/2020), Delta (T478K), and Omicron (BA.1) variants were analyzed by Fourier transform ion cyclotron (FTICR)-MS and trapped ion mobility spectrometry (TIMS)-MS. To elucidate the molecular sequence and O-glycans of the various S-RBDs, N-glycans from the S-RBD were removed by using a PNGase F treatment to minimize the interference posed by N-glycan heterogeneity.
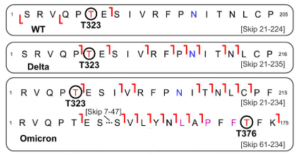
Detailed top-down MS/MS analysis of the S-RBD O-glycoforms revealed the presence of a new O-glycosite (Thr376) unique to the Omicron variant. Fascinatingly, all detected S-RBD O-glycans for the WT and Delta variants were confidently assigned solely to Thr323. Given the smaller number of mutations present on Delta as compared to Omicron, it is not surprising that the O-glcyosite Thr323 was conserved between Delta and WT variants.
On the other hand, the Omicron variant yielded both the familiar Thr323 O-glycosite and a new Thr376 O-glycosite corresponding to the GalNAcGal(NeuAc)2 O-glycoform that is simultaneously occupied. This Thr376 O-glycosite is conveniently n + 3 adjacent to a proline at residue 373, which is consistent with previous reports of increased O-glycosylation frequency near proline. This particular Pro373 is a site-specific mutation unique to the Omicron variant and likely is the reason for this new O-glycosite. It might be better to note that the site occupancy of the Thr376 site was low(< 30%) relative to the Thr323.
However, it is unclear at this moment if Thr376 O-glycan has any relationship with Omicron infectivity and escape from immunological protection.