A drought-tolerant soil for wheat by adding silica particles with soil bacteria producing exopolysaccharides effectively
A group from Swedish University of Agricultural Sciences, Uppsala, Sweden, etc. has developed a drought-tolerant soil for wheat by adding silica particles with soil bacteria producing exopolysaccharides effectively.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8229586/
Agriculture faces several challenges at the global level and, in coming decades, drought is expected to expand globally owing to increased evaporation and reduced rainfall, as well as or changes in the spatial and temporal distribution of rainfall. The scientific community across the world is earnestly looking for novel solutions to enhance crop plant stress tolerance under limited resource availability, and several environmentally friendly solutions have shown huge potential but need to be optimized for wide-scale field application. One such solution includes strengthening plants’ natural defence systems with plant growth promoting rhizobacteria. Predicting and controlling the rhizosphere has the potential to harness plant microbe interactions and restore plant ecosystem productivity, improve plant responses to environmental stress, and mitigate the effects of climate change.
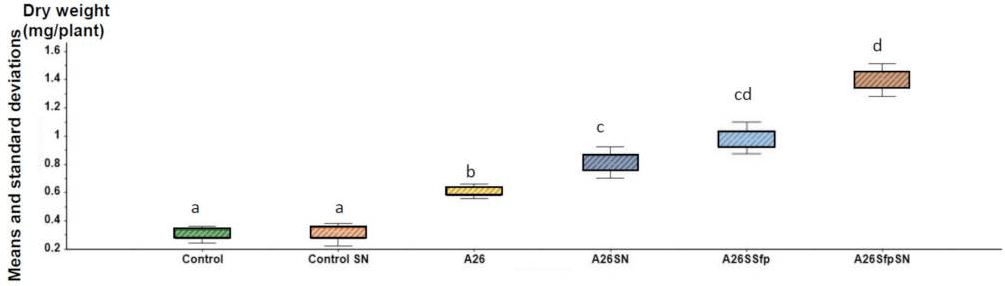
First of all, soil bacteria named A26Sfp was created from its wildtype A26 by genetically silencing 4-phosphopantetheinyl transferase to improve drought tolerance ability. A26Sfp, compared to its wildtype A26, is enhanced in biofilm exopolysaccharides production consisting D-glucuronate. Polysaccharides are basically hydrophilic, and therefore are suitable to increase water retention.
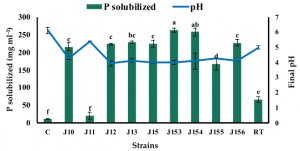
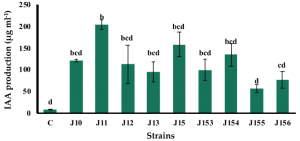
Strains A26, A26Sfp were grown in 1/2 Tryptic soy broth (TSB) with 50 µg/mL silica particles (SN) at 30 ± 2 °C for 24 h. While the silica particles did not have a significant impact on the bacterial number, silica particles improved A26 and A26Sfp exopolysaccharides production by 46% and 29%, respectively. A26Sfp EPS production was 30–40% higher than its wildtype and A26Sfp SN treatment caused a further 20% increase in exopolysaccharides production. Why silica particles enhanced exopolysaccharides production was not clearly explained. However, morphological changes observed, bacterial elongation and cell aggregate formation, might reflect some changes in exopolysaccharides production capability.
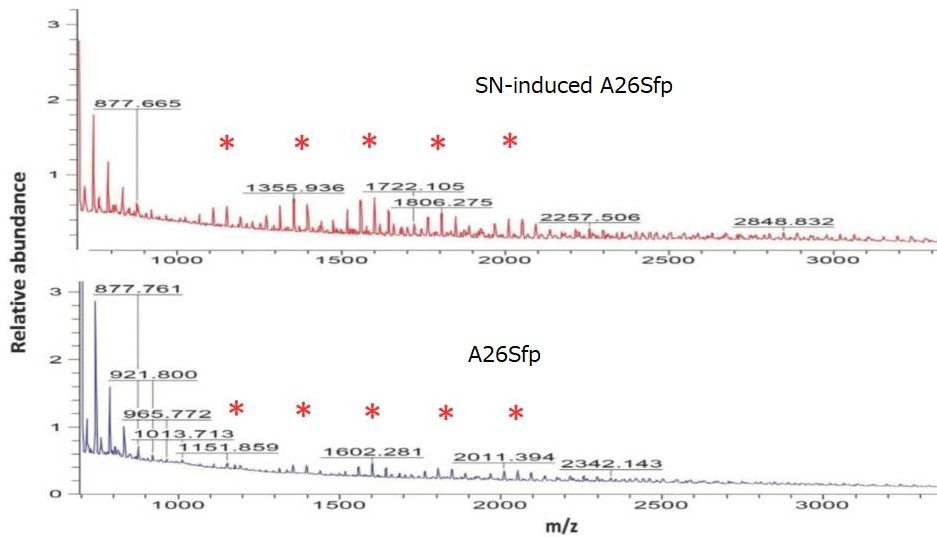
Quantification of exopolysaccharides using mass spectroscopy showed that the oligosaccharides produced by A26Sfp with SNs had longer chains than that of A26 wild type, suggesting that the improvement of the drought-tolerant soil will be due to increased production of longer chain polysaccharides.