Galectin-3 could be a GOOD prognostic marker against severe COVID-19
A group from University of Medicine “Aldo Moro”, Bari, Italy, etc. has reported on prognostic power of Galectin-3 against severe COVID-19.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8332745/
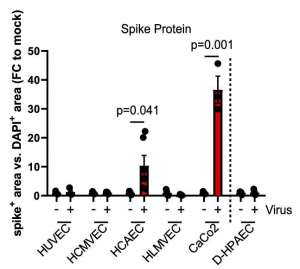
This is the first study in scientific literature assessing the prognostic role of Galectin-3 in acute respiratory failure secondary to COVID-19 disease. Patients with higher serum levels of Galectin-3 tend to develop a more severe degree of ARDS with a worse prognosis. It is well known that SARS-COV2 infection can lead to the so called “cytokine storm” in some susceptible patients. For instance, our non-survivors group shows increased blood levels of various inflammation markers, which are frequently associated with negative outcomes in COVID-19 disease. Nevertheless, only IL-6, CRP and Galectin-3 remain statistically significant in our multivariate regression model. This finding is not surprising for IL-6 and CRP, which were previously reported as important prognostic markers in COVID-19 disease. On the contrary, this is the first study addressing this role for Galectin-3. Furthermore, among the explored parameters, Galectin-3 shows the best AUC curve in ROC analysis, showing good diagnostic power for severe ARDS (AUC 0.75, p = 0.001) using a cut-off value of 35.3 ng/ml.
In fact, patients with Galectin-3 serum levels above 35.3 ng/ml were not only more prone to develop severe ARDS, but also markedly at higher risk of ICU admission or death. The cohort size of this study was 156 patients.