A group from University of Oxford, UK, etc. has reported on mapping of SARS-CoV-2 spike glycoprotein-derived antigens presented by HLA class II on dendritic cells.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8116342/
It is very curious for blog admin whether HLA-II bound glycopeptides presented as SARS-CoV-2-specific antigens on dendritic cells have the same glycosylation as phagocytized SARS-CoV-2 by dendritic cells.
HLA-II-bound glycopeptides were identified from 14 N-linked glycosylation sites in Spike from total 22 glycosylation sites. HLA-II-bound peptides carried predominantly short paucimannosidic-type N-glycans while original Spike carried oligomannosidic- and GlcNAc-capped complex-type N-glycan structures at these sites. The paucimannosylation of the HLA-II-bound peptides comprised both core-fucosylated and fucosylated species. This reveals there is substantial trimming of glycan residues on the glycopeptides during antigen processing in dendritic cells.
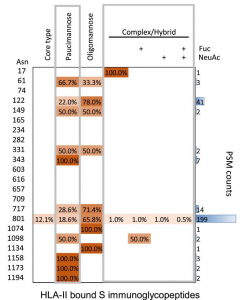
The heatmap colors in a figure above indicate the relative frequency of each glycan composition present, and the total number of peptide spectral matches (PSM) is also shown (blue bars).
