A group from National Cancer Institute, Frederick, USA, etc. has reported good Serum cytokine and chemokine markers associated with effective immune response to SARS-CoV-2 in mRNA vaccination.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8299183/
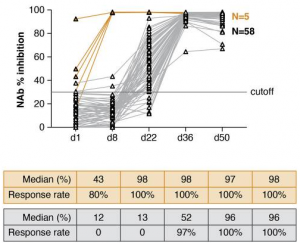
A cohort of 63 health-care workers received the BNT162b2 mRNA vaccine and was monitored for the development of neutralizing antibodies. There were two types of recipients in this cohort. One is individuals without any SARS-CoV-2 infection before the vaccination (58 recipients), and the other was individuals with pre-existing SARS-CoV-2 infection (6 recipients). The 58 recipients showed immune responses first detected 3 weeks after the 1st dose (day 22), which was followed by a significant increase after the 2nd dose by day 36. The neutralizing ability was measured using a surrogate virus neutralization test, and an assay that reached a median of 96% inhibition after the 2nd dose, In contrast, the 5 recipients with pre-existing SARS-CoV-2 immunity showed antibody responses to SARS-CoV-2 at the day of vaccination, followed by an immediate strong anamnestic response after the 1st dose (day 8). The antibody responses did not further increase upon the 2nd vaccination and remained significantly higher than those in the SARS-CoV-2-naive vaccine recipients.
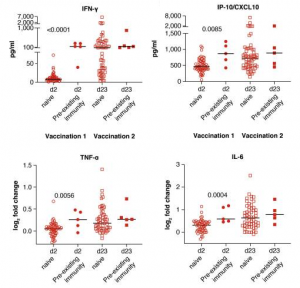
This study highlights important associations of several immunoregulatory molecules induced by vaccination with innate and adaptive immune responses elicited by an mRNA-based vaccine. The early cytokine/chemokine signature featuring IL-15, IFN-γ, and IP-10/CXCL10 may be used to monitor effective vaccination and as a guide to optimize the efficacy of mRNA vaccination strategies.