Farmed minks were infected with SARS-CoV-2 from humans: A future threat with new virus variants
A group from University Göttingen, etc. has alarmed a possibility in reinfection of mutated SARS-CoV-2 in farmed mink to human.
https://pubmed.ncbi.nlm.nih.gov/33857422/
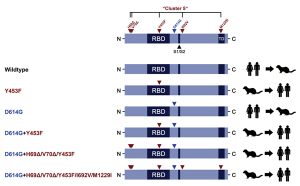
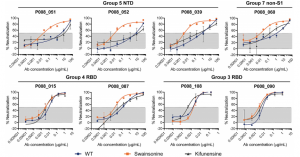
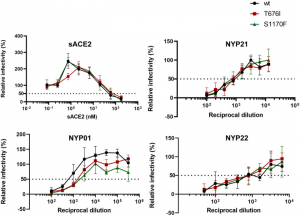
Transmission of SARS-CoV-2 from humans to farmed mink has been observed in Europe and USA. Substitution D614G, which is dominant in SARS-CoV-2 from humans, was also found in viruses from mink, and a mink-specific mutation Y453F (mutant D614G+Y453F) or Y453F in conjunction with H69Δ, H70Δ (mutant D614G+H69Δ/H70Δ/Y453F) were also found in mink. The Y453F mutation locates in RBD, and reduced inhibition by human serum samples tested. The neutralizing titer IC50 increased by 1.62 times with this mutation, and the neutralizing titer of REGN10933, which is one component of a cocktail therapy (REGN-COV2), also increased with this mutation.
New emerging variants generated through transmission from humans to wild animals could represent a future threat to human health.