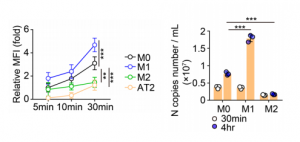
Maackia amurensis lectin reduces the expression levels of ACE2, ADAM17, Furin, etc. : A new finding in suppressing SARS-CoV-2 infection
A group from Rowan University, Stratford, USA, etc. has suggested that Maackia amurensis lectin (MAL, MAA, MASL as abbreviated names) could be effctive in inhibiting SARS-CoV-2 infection.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8019238/
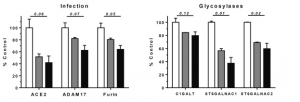
Oral squamous cells were used as a model cell in this study. Transcriptome analysis was done to investigate effects of MAL onto ACE2, ADAM17, Furin, and Glycosyltransferases (GalNAc-T, ST6GalNAc-1, and ST6GalNAc-2).
Interestingly, it was shown that those decreased in a MAL dose dependent manner. For instance, at a dose of 1925nM of MAL, ACE2 mRNA level decreased by 60%, ADAM17 by 40%, and ST6GalNAc-1 by 60%. As a result of these events, MAL decreases inflammatory signaling events that would otherwise lead to activation of the IL6 amplifier implicated in COVID-19 induced ARDS
MAL is known to have binding specificity to α2-3Sia.