A group from Department of Protein Engineering, Faculty of Biotechnology, University of Wroclaw, Wroclaw, Poland has reported that galectin-1, -7, and -8 can activate FGFR1 signaling and control endocytosis.
https://biosignaling.biomedcentral.com/articles/10.1186/s12964-024-01661-3
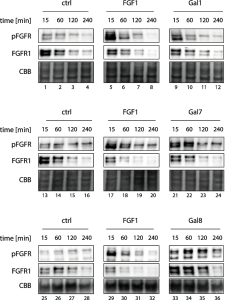
N-glycans of FGFR1 are recognized by extracellular galectins (Gal-1, Gal-7, and Gal-8), which are not authentific ligand of FGFR1 (i.e., FGF1), and the binding of those galectins to FGFR1 trigers activation of the receptor and initiation of downstream signaling cascades. Subsequent endocytosis of activated FGFR1 serves as a major cellular mechanism for the downregulation of FGFR1 signaling.
Both FGF1 and Gal-1 directly activate FGFR1 and after short and intensive pulse of FGFR1 signaling, the receptor is shut down due to the induction of clathrin medited endocytosis, followed by lysosomal degradation of the receptor. Gal-7 and -8 also directly activate FGFR1 by the receptor clustering mechanism, but by inhibiting FGFR1 endocytosis and degradation, these galectins largely prolong FGFR1 signaling.