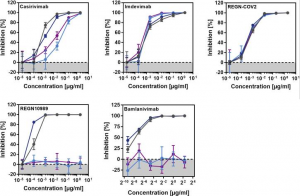
A polyphenol from green tea (GCG) could inhibit SARS-CoV-2 replication effectively
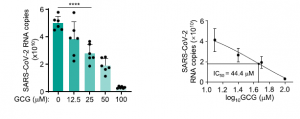
A group from Fudan University, etc. has shown that Gallocatechin gallate (GCG), a polyphenol from green tea, inhibits SARS-CoV-2 replication efficiently.
https://www.nature.com/articles/s41467-021-22297-8
SARS-CoV-2 N protein is a structural protein binding to RNA, and form a shell embracing SARS-CoV-2 RNA. This is a typical example of so called Liquid-Liquid Phase Separation (LLPS) which is a mechanism in organizing macromolecules such as proteins and RNAs into membrane-less oil droplet like organelles.
Authors has shown that GCG could could inhibit N protein LLPS in the context of SARS-CoV-2 infection effectively. Since, the amino acid sequence shares ~90% homology among corona viruses, targeting N protein by GCG could be a novel drug candidate not only for SARS-CoV-2 but also for new coronaviruses in the future.