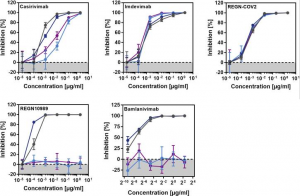
A group from German Primate Center, Göttingen, etc. has reported on the effectiveness of major monoclonal antibodies for COVID-19 (Casirivimab, Bamlanivimab, Imdevimab)against SARS-CoV-2 variants, that of a cocktail monoclonal antibody (REGN-COV2: consisting of Casirivimab and Imdevimab), and also that of Pfizer BNT162b2 vaccine against those variants.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7980144/
A cocktail monoclonal antibody (REGN-COV2)efficiently inhibited infection mediated by the S proteins of all variants (B.1.1.7, B.1.351, P.1). However, infection mediated by the S proteins of the B.1.351 and P.1 variant was completely resistant to REGN10989 and Bamlanivimab.
On the other hand, the Pfizer BNT162b2 vaccine is based on an mRNA that encodes for the SARS-CoV-2 S protein and is said to be highly protective against COVID-19. All sera from 15 donors immunized twice with BNT162b2 efficiently inhibited entry driven by the WT S protein and inhibition of entry driven by the S protein of the B.1.1.7 variant was only slightly reduced. However, 12 out of 15 sera showed a markedly reduced inhibition of entry driven by the S proteins of the B.1.351 and P.1 variants