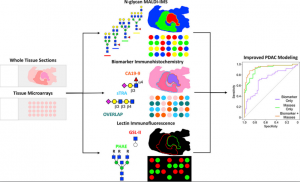
Glycan markers for pancreatic duct adenocarcinoma
A group from Medical University of South Carolina etc. has reported their findings on glycan markers for pancreatic duct adenocarcinoma.
https://www.mcponline.org/article/S1535-9476(20)35126-4/fulltext
In order to study the glycan markers of pancreatic duct adenocarcinoma in detail, MS (MALDI-FTICR, MALDI-QTOF), antibody immunostaining (CA19-9, TRA-1-60), lectin staining (PHA-E, GSL-II) were used.
To summarize the results, pancreatic duct adenocarcinoma has an increase in the structure of α2-3 Sia, poly-LacNAc, branching, bisecting GlcNAc, core fucose, and terminal GalNAc compared to normal tissues. As for Sia, α2-3Sia is mainly expressed in tumor stroma regions, and α2-6Sia is slightly stronger in adenocarcinoma regions.