Electrochemical high sensitivity detection using RCA isothermal amplification and Redox reaction of the novel coronavirus (SARS-CoV-2)
RT-PCR is the Gold Standard for the detection of the new coronavirus (SARS-CoV-2).
A group from Mahidol University, Nakhon Pathom, Thailand etc. has reported an electrochemical detection method that has the same sensitivity and specificity as RT-PCR and do not require expensive devices.
https://www.nature.com/articles/s41467-021-21121-7
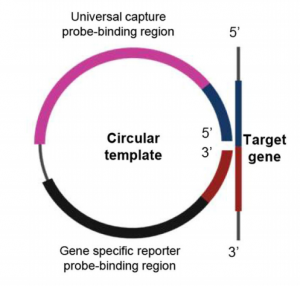
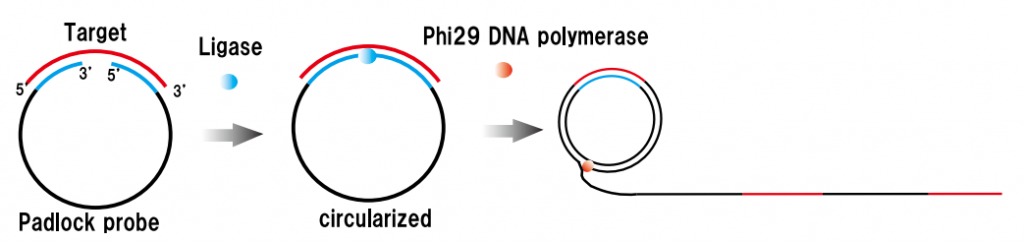
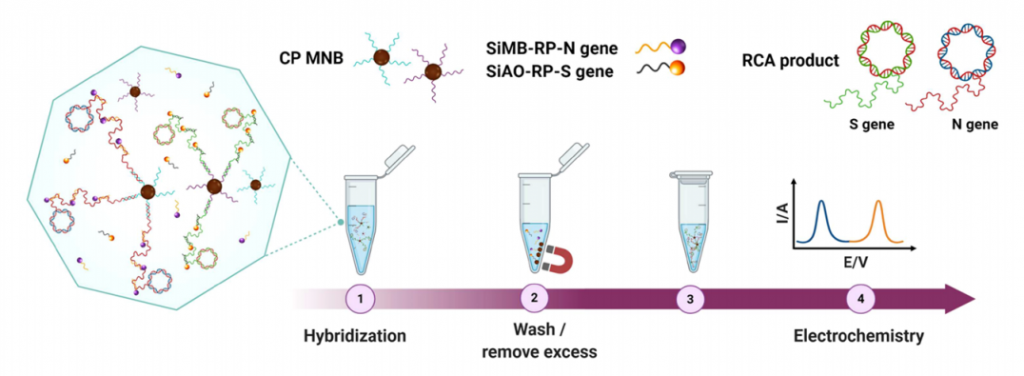
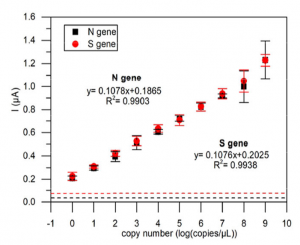
In order to detect SARS-CoV-2 with high sensitivity, of course, a gene amplification process is required. They adopted Rolling Circle Amplification (RCA). The RCA method, like the PCR and LAMP methods, has the advantage that carryover contamination has a small impact on the next amplification process and can be amplified in an isothermal bath at around room temperature (25°C to 37°C). The RCA amplicon (Padlock probe) was designed as shown below and was consist of Target gene specific to the target you want to detect (N-gene, S-gene, etc. of SARS-CoV-2), Universal capture probe-binding region, and Generic reporter probe-binding region. With this Padlock probe, RCA results in long DNA amplicons containing hundreds of tandem repeats of the Padlock DNA complementary sequence. For this RCA products, hybridize probe-conjugated magnetic bead particle (CP-MNB) and Silica methylene blue reporter probe (SiMB-RP) to obtain the final product. Methylene blue or Acridine orange was used to as a redox regent. The current obtained by the electrochemical reaction increased with copy numbers as shown below. As a result, the detection limit sensitivity reached 1 copy/uL.