Evaluation of glycan structure of reference mAb (humanized IgG Type 1) in NIST, U.S.A.
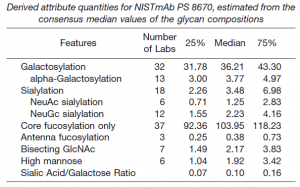
NIST in the U.S. offers humanized IgG Type 1 mAb as a reference mAb. NS0 cells are used to manufacture this mAb. NS0 cell is a model cell line derived from non-secretory mouse myeloma, which is commercially used in biomedical research and the production of therapeutic proteins.
https://pubmed.ncbi.nlm.nih.gov/31591262/
The status of glycan addition is reported by comprehensively following the results of 103 evaluations conducted at 73 institutions around the world. It is likely to be useful as glycan modification information of reference mAb of IgG Type 1.