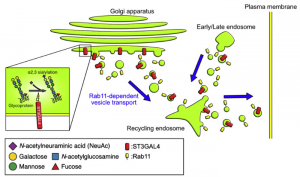
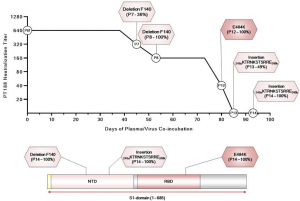
Vitamin C and the new coronavirus (COVID-19)
A group from University of Helsinki etc. has reported on the relationship between Vitamin C and COVID-19.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7848027/
In healthy people, a Vitamin C intake of about 0.1 g/day is enough to maintain the concentration of Vitamin C in the blood. However, when infected with COVID-19 and becomes severe, the concentration of Vitamin C in the blood decreases sharply, and it can be reduced to the same level as vitamin C deficiency and further to a level that is almost undetected.
Taking a large amount of Vitamin C (6-8 g/day) has the following effects:
Reduced ICU treatment duration by an average of 8%
Reduced fatality from 35% to 78%
Since there are no side effects of Vitamin C, this is also considered to be effective for the COVID-19 treatment.