A group from University of Melbourne, etc. has investigated changes in glycan modification in myogenesis over time, while studying the functions of Galectins.
https://www.mcponline.org/article/S1535-9476(20)35144-6/fulltext
In myogenesis, there was a tendency that terminal di-Gal modification down regulated, α2-6Sia modification up regulated, α2-3Sia modification down regulated inversely, and paucimannose up regulated. These changes were thought to be related to intercellular signaling in myogenesis, but the specific signal paths were still unknown. On the other hand, as for galectins, the expression of Galectin-1 increased and the expression of Galectin-3 decreased.
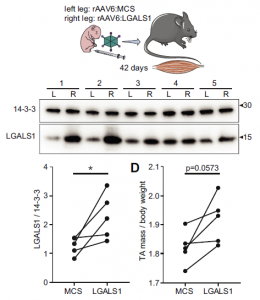
Using newborn mice, an empty multiple cloning site (MCS) was injected with AAV6 into the left foot (as a control), and a galectin-1 gene (LGALS1) was injected with AAV6 into the right foot, and the difference between them was compared after 42 days. it was found that muscle mass was significantly increased with the injection of LGALS1. So, it is clear that galectin-1 is able to promote muscle development.
In the figure below, 14-3-3 protein was referenced as a comparison of LGALS1. 14-3-3 protein is related to intercellular signaling, and recognizes Ser/Thr residues in a specific domain of target protein with a phosphorylation-dependent way, and extracts the physiological function of the phosphorylation state.