A group from Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China, etc. has reported about the crystal structure of Cry78Aa from Bacillus thuringiensis.
https://www.nature.com/articles/s42003-022-03754-6
Biological control methods using Bacillus thuringensis (Bt) as well as genetically modified plants expressing insecticidal proteins from Bt have been proven effective and economic against some insect pests. Cry78Aa is a novel protein identified from the Bt C9F1 strain that effectively kills rice planthoppers, with median lethal concentration (LC50) values against Laodelphax striatellus and Nilaparvata lugens of 6.89 and 15.78 μg ml−1, respectively. The activity of Cry78Aa does not require in vitro activation or any additional components, making it convenient for application in field trials.
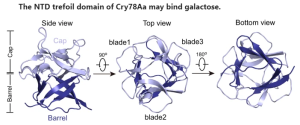
In this paper, the crystal structure of Cry78Aa was analyzed in detail. This structure consists of two independent domains: a trefoil domain at the N-terminus, which shares the highest identity with S-type lectin, and a pore-forming domain belonging to the aerolysin family. Bioassays showed that the NTD or CTD of Cry78Aa alone has no toxicity against planthopper nymphs, indicating that its insecticidal activity is dependent on the cooperation of both domains. The NTD of Cry78Aa plays a vital role for its insecticidal activity, probably by recognize galactose derivatives linked to proteins or lipids on the surface of the cell membrane.