A group from Harvard Medical School, USA, etc. has reported that as many as 10% of circulating monocytes and 8% of lung macrophages in COVID-19 patients were infected with SARS-CoV-2, and FcR-mediated uptake of antibody-bound virus triggers inflammatory death (pyroptosis), which is really a double-edged sword in COVID-19.
https://www.researchsquare.com/article/rs-153628/v1
How SARS-CoV-2 triggers inflammation is not clearly understood.
Monocytes and macrophages are sentinel immune cells in the blood and tissue, respectively. These immune cells sense invasive infection, and form inflammasomes that activate caspase-1 and gasdermin D (GSDMD) pores leading to pyroptosis and release of inflammatory cytokines.
Monocytes are generally thought not to express ACE-2. Indeed, ACE-2 was not detected or barely detected by flow cytometry and qRT-PCR on both healthy donor (HD) monocytes, even when they were activated by SARS-CoV-2. Monocytes express 2 Fcg receptors – CD32 (FcgRII, expressed on most blood monocytes) and CD16 (FcgRIII, expressed on a small minority of blood monocytes that are activated and increased in number in COVID-19 patients). These receptors could recognize antibody-opsonized viral particles and mediate their entry via antibodydependent phagocytosis (ADP).
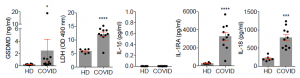
Release of large proteins such as the tetramer LDH, is a pathognomonic feature of pyroptosis and other forms of necrotic cell death. Elevated LDH was one of the best correlates of severe COVID-19 disease. Plasma levels of GSDMD, LDH, IL-1RA and IL-18, were all elevated in severe patient samples compared to those with mild or moderate disease. And also, GSDMD eQTLs were most significantly associated with increased COVID-19 respiratory failure.
After virus uptake, SARS-CoV-2 begins to replicate in monocytes, as evidenced by detection of double-stranded RNA etc. However, infection is aborted, and infected cells undergo inflammatory cell death (pyroptosis) mediated by activation of the NLRP3 and AIM2 inflammasomes, caspase-1 and GSDMD. Moreover, tissue-resident macrophages from COVID-19 lung autopsy specimens showed similar evidence of inflammasome activation. These findings taken together suggest that antibody-mediated SARS-CoV-2 infection of monocytes/macrophages triggers inflammatory cell death that aborts production of infectious virus but causes systemic inflammation that contributes to severe COVID-19 disease pathogenesis.