A group from Univ. Grenoble Alpes, France, etc. has reported glycan binding specificity of Scedosporium apiospermum Lectin 1 (SapL1).
https://www.nature.com/articles/s41598-021-95008-4
Scedosporium apiospermum is an emerging opportunistic fungal pathogen responsible for life-threatening infections in humans. Host–pathogen interactions often implicate lectins that have become therapeutic targets for the development of carbohydrate mimics for antiadhesive therapy. Here, we present the first report on the identification and characterization of a lectin from S. apiospermum named SapL1.
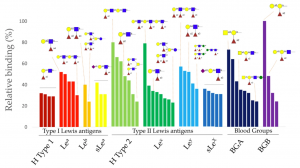
To identify the potential ligands of SapL1 on lung epithelial cell surfaces, SapL1 was applied onto the glycan array version 5.4 of the Consortium for Functional Glycomics (USA) consisting of 585 mammalian glycans. It was labelled with Fluorescein Isothiocyanate (FITC) and its binding properties were analysed at two different concentrations (5 and 50 μg/mL). SapL1 recognizes fucosylated oligosaccharides independently of the fucose linkage. The α1,2 and α1,3/4 linked fucosides displayed the highest affinity whilst the lowest was seen with the α1,6 linked ones. The weakest interactions with fucosylated compounds were reported for branched oligosaccharides.
Analysis of the glycans constituting blood group determinants revealed that SapL1 binds to all epitopes with a preference for H type 2 blood group (Fucα1-2Galβ1-4GlcNAcβ) then Lewis a (Galβ1-3(Fucα1-4)GlcNAcβ) and Lewis X (Galβ1-4(Fucα1-3)GlcNAcβ). However, most of the recognized branched oligosaccharides contained the core fucose Fucα1–6. Epitopes with two fucose units, such as Lewis b and fucosylated polylactosamine, were also well recognized. Addition of α-galactose or α-GalNAc as in blood group B or A antigens did not impair Fucα1-2 recognition.