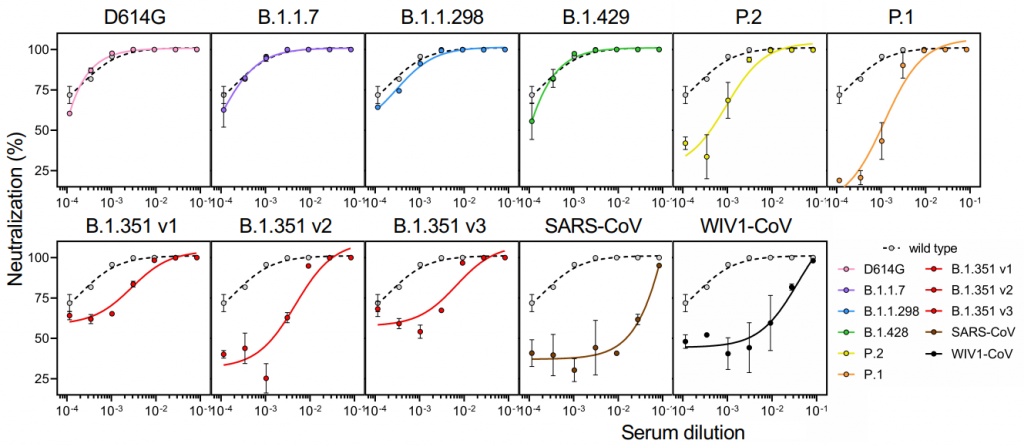
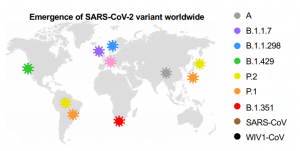
Effectiveness of Pfizer and Moderna vaccines for various SARS-CoV-2 variants occuring in the world: B.1.351 and P.1 variants are critical
A group from Massachusetts General Hospital, etc. has reported on effectiveness of two typical vaccines, BNT162b2 (Pfizer) and mRNA-1273 (Moderna), for various SARS-CoV-2 variants occuring in the world.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7899476/
Distribution of typical SARS-CoV-2 variants in the world.
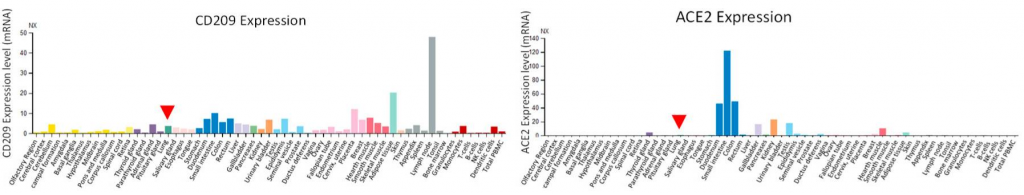
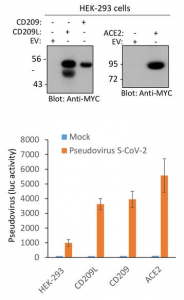
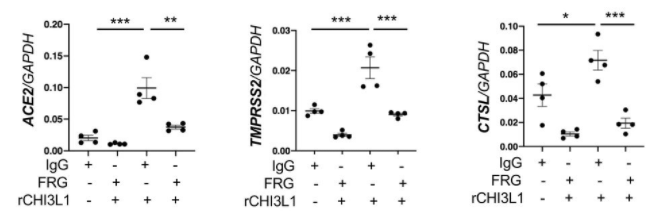
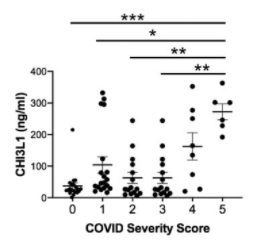
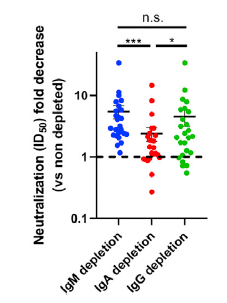
Effectiveness of Pfizer and Moderna vaccines is shown by the neutralization response. There seems to be almost no change for B.1.1.7 variant, but the neutralization activity of these vaccines are significantly reduced in B.1.351 v2 and P.1 variants. Two common mutations, K417N and E484K, exist in B.1.351 and P.1 variants, and these mutations do not exist in B.1.1.7.