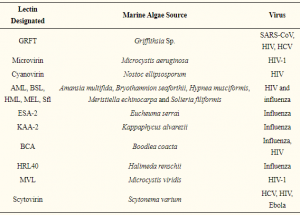
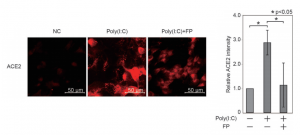
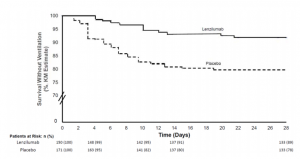
Alterations in the dendritic cells (DCs) signal paths after α2-3Sia binding: α2-3Sia induces anti-inflammatory actions
A group from Vrije Universiteit Amsterdam, Netherlands has studied alterations in the dendritic cells (DCs) signal paths after α2-3Sia binding.
https://www.frontiersin.org/articles/10.3389/fimmu.2021.673454/full
DCs possess the extraordinary capacity to recognize various pathogens with its pattern recognition receptors (PPRs) and present those antigens with MHC to tell the information to T-cells.
Sialic acids are increasingly attracting attention with their role in the immune regulation of cancer. During cancer progression, tumor cells often highly increase their sialic acid expression to create an immunosuppressive tumor microenvironment. Sialic acids are also advantageous for pathogens to evade from immune attacks. Bacteria obtain sialic acids by de novo synthesis or from an environmental source, and thereby can hide and escape from immune surveillance.
Authors analyzed alterations in the DC phosphoproteins, Kinase signatures, and JAK-STAT signaling pathway due to α2-3Sia stimulation in the presence of Lipopolysaccharide (LPS). LPS is often used as a model antigen (as an endotoxin) to activate immunity.
Overview of the result was summarized as follows.
Phosphorylation was enhanced upon α2-3Sia stimulation, while simultaneous α2-3sia and LPS stimulation resulted in less phosphorylation, indicating that recognition of a2-3Sia by DC alters TLR 4 triggering and DC signaling. α2-3Sia stimulated DCs compared to control with simultaneous α2-3sia and LPS stimulation showed decreased scoring of kinases ERK, AKT1, PKCB, GSK3, PKCD, PAK1, PKA, GSK3, GRK, IκB, and RAF1, and those affected kinase signatures were involved in the chemokine signal pathway. α2-3sia stimulation affected the JAK-STAT signaling by lowering the phosphorylation status of STAT3 and STAT5A.
Although the things are so complicated, these changes result in downregulation of IL-12 (inflammatory cytokine) pathway and inversely upregulation of IL-10 (anti-inflammatory cytokine) pathway, leading allover features to the direction of suppressing inflammation.