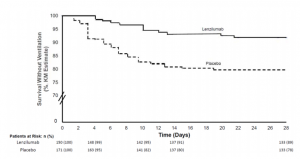
A group from Mayo Clinic, etc. has reported a promising result of a phase 3 clinical trial of Lenzilumab against COVID-19.
https://pubmed.ncbi.nlm.nih.gov/33972949/
In COVID-19, it has been known that high levels of granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting T-cells are associated with disease severity, myeloid cell trafficking to the lungs, and ICU admission. GM-CSF is a kind of cytokine which activates differentiation of myeloid cells, leading to elevations of downstream inflammatory chemokines (MCP-1, IL-8, IP-10) and cytokines (IL-6, IL-1).
Lenzilumab is a novel anti-human GM-CSF monoclonal antibody that directly binds GM-CSF and prevents signaling through its receptor. It has high binding affinity (25 pM) for glycosylated human GM-CSF and a slow off-rate. A phase 3 randomized, double-blind, placebo-controlled clinical trial was designed to demonstrate the effectiveness of lenzilumab. The dose of lenzilumab was 600mg and administered 8hours apart for 28 days.
Lenzilumab could improve the likelihood of survival without ventilation (SWOV) through Day 28 by 54%. It should be noted that the improvement in SWOV was most evident in patients with CRP<150 mg/L who are less than 85 years of age.