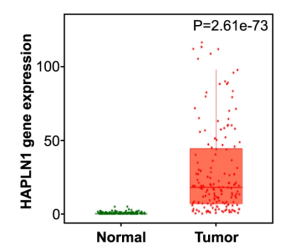
A group from Division Vascular Signaling and Cancer, German Cancer Research Center (DKFZ), Heidelberg, Germany, etc. has reported that Proteoglycan link protein-1 (HAPLN1) would be a driver for peritoneal metastasis in Pancreatic ductal adenocarcinoma (PDAC) and could be a GOOD prognostic marker.
https://www.nature.com/articles/s41467-023-38064-w
Cellular plasticity is an important feature of tumore cells. Cellular plasticity is characterized by the ability of cells to convert between different (intermediate) cellular states by inducing epithelial-to-mesenchymal transition (EMT), as well as mesenchymal-to-epithelial transition (MET) and features of cancer cell stemness5. Such plasticity not only makes cancer cells more prone for invasion and adaption to the microenvironment, but also protects them from apoptosis, immune attack and chemotherapy.
A key regulator of metastasis is the microenvironment that tumor cells are facing during their journey to the metastatic site. The tumor microenvironment (TME) consists of several cellular and non-cellular components, including cancer-associated fibroblasts (CAFs), endothelial cells, immune cells and extracellular matrix (ECM). In PDAC, the TME is strongly desmoplastic, with substantial accumulation of ECM components. Hyaluronic Acid (HA), which is one major component of the ECM, facilitates tumor progression and metastasis through promoting partial EMT, invasion, immunomodulation and therapy resistance. CAFs are the main producers of ECM.
HAPLN1 is a HA and chondroitin sulfate proteoglycan (CSPG) crosslinker in the ECM, but its role has been so far poorly understood in cancer. In this study it was identified that HAPLN1 was the most upregulated genes in PDAC compared to adjacent tissue. It was thought that HAPLN1 could be defined as a mediator of peritoneal dissemination in PDAC, by inducing a highly plastic phenotype in cancer cells, which leads to a pro-tumoral metastatic niche.