A group from Department of Chemistry, Georgia State University, Atlanta GA USA, etc. has reported about lectins specific to bisecting GlcNAc.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9241959/
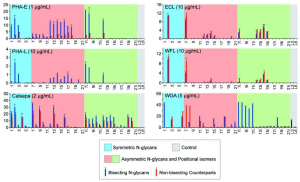
Many plant lectins could tolerate the bisecting GlcNAc, among which the bindings of PHA-E and Calsepa were enhanced. It was observed that even at a low concentration (1 μg mL−1), PHA-E and Calsepa showed bindings to non-bisected N-glycans, which would pose a significant problem in their wide applications of bisected glycan identification and cancer biomarker discovery. Surprisingly, PHA-L exhibited specific recognition of bisected biantennary N-glycans, which could find promising implementation in probing such structures, e.g., in antibodies, where β1-6-branched glycans are absent.
However, it should be noted that PHA-L has a strong specificity for tri/tetra antennary N-glycans.