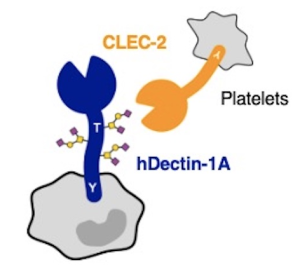
A group from Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan, etc. has reported that Human Dectin-1 is O-glycosylated and serves as a ligand for C-type lectin receptor CLEC-2.
https://pubmed.ncbi.nlm.nih.gov/36479973/
Dectin-1 is known as the best-characterized C-type lectin receptors (CLRs) recognizing β-glucan on pathogens. Here, it was first found that Dectin-1 could be a ligand for CLEC-2, another CLR expressed on platelets. It was also known that CLEC-2 recognizes its endogenous ligand podoplanin in an O-glycosylation-dependent manner.
Dectin-1 is a mucin-like protein and its stalk region is highly O-glycosylated with sialylated core 1 (Galβ1-3GalNAc) and/or sialylated core 3 (GlcNAcβ1-3GalNAc) glycans. This finding will be the first example of an innate immune receptor also functioning as a physiological ligand to regulate ontogeny upon glycosylation.