A group from Faculty of Pharmacy and Biochemistry, University of Zagreb, Zagreb, Croatia, etc. has reported that plasma high-mannose glycan change of complement C3 showed notable discriminative power between children with early onset type 1 diabetes and their healthy siblings with AUC of 0.879.
https://www.mcponline.org/article/S1535-9476(22)00215-8/fulltext
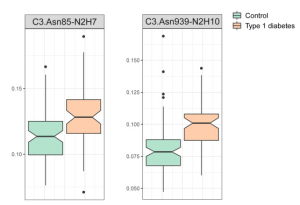
Children’s type 1 diabetes was associated with an increase in the proportion of high-mannose structures of complement C3 with more mannose units.
In this experiment, high-mannosylated C3 was enriched by ConA lectin, and the glycan structures were analyzed by LC-MS/MS. C3 has two glycosylation sites at Asn85 and Asn939, and the high-manosylated structure gets longer with developing type 1 diabates as shown below.