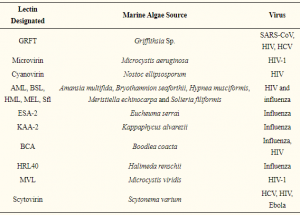
POCT biosensor (named RAPID) for SARS-CoV-2 based on electrochemical reading with quite high sensitivity and high detection speed
A group from Perelman School of Medicine, University of Pennsylvania, etc. has reported a POCT biosensor (named RAPID) for SARS-CoV-2 based on electrochemical reading with quite high sensitivity and high detection speed.
https://pubmed.ncbi.nlm.nih.gov/33997767/
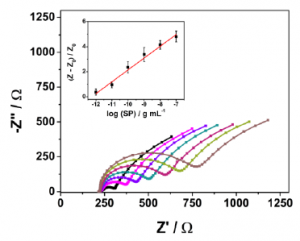
ACE2 was used as a probe detecting SARS-CoV-2 Spike protein. Enzyme immobilization on the electrode was done by cross-linking ACE2 using the bifunctional chemical cross-linker glutaraldehyde. BSA was used to block the electrode’s surface after immobilization of ACE2. Nafion was added to increase the sensitivity further.
The binding between two molecules (ACE2 and SARS-CoV-2 Spike protein) causes a change in interfacial electron transfer kinetics between the redox probe, ferricyanide/ferrocyanide in solution and the conducting electrode sites. This electrochemical change is then detectable by monitoring the charge-transfer resistance.
As a result, a linear concentration range from 10 fg/mL to 100 ng/mL was obtained (R2 = 0.993) and limits of detection (LOD) and quantification (LOQ) were calculated as 2.18 fg/mL on signal to noise ratios (S/N=3). With RAPID, result is obtained in 4 minutes (2 minutes of sample incubation + 2 minutes to perform the EIS analysis)..
In blinded tests using 139 nasopharyngeal swab samples, 109 of which were COVID-19 positive and 30 COVID-19 negative as determined by RT-qPCR, RAPID demonstrated high sensitivity, specificity and accuracy for nasopharyngeal (83.5%, 100% and 87.1%, respectively) and saliva (100%, 86.5% and 90.0%, respectively) samples.