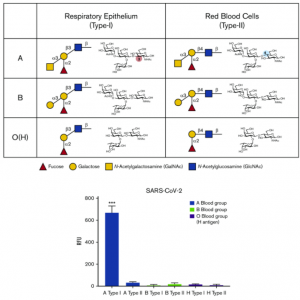
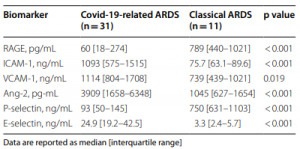
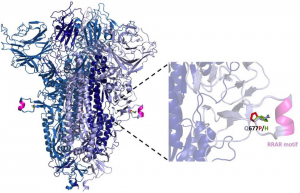
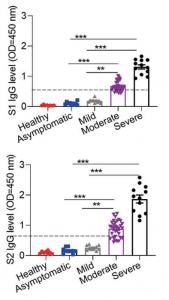
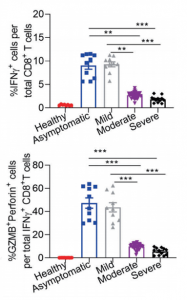
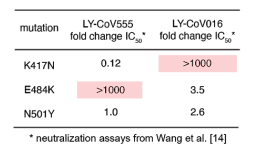
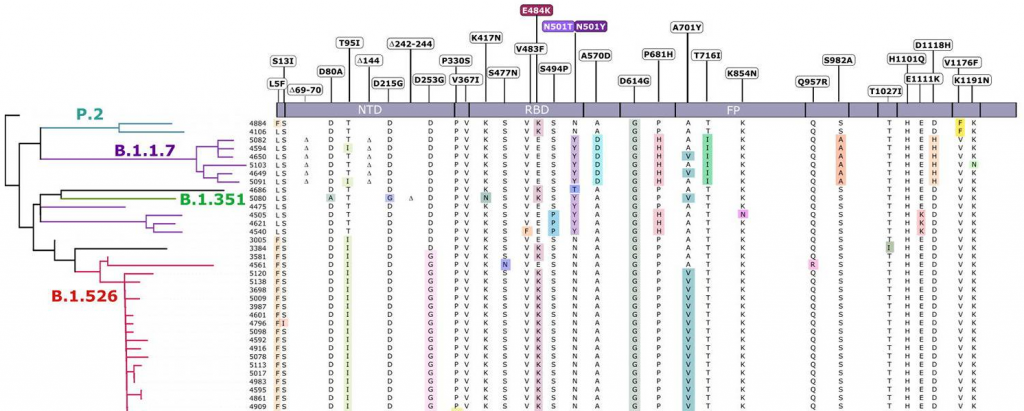
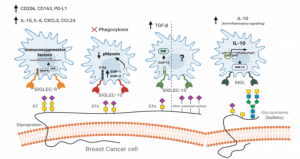
A counter-receptor of Siglec-7 was identified as leukosialin (CD43)
A group from Nagoya Univ. has identified a counter-receptor of Siglec-7.
https://www.jbc.org/article/S0021-9258(21)00251-9/fulltext
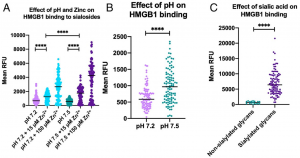
Siglec-7 binds to sialic acids and are expressed on natural killer (NK) cells and monocytes. Siglec-7 suppress cytotoxicity of NK cells through interaction with a counter-receptor on it. However, the counter-receptor of Siglec-7 has not been identified yet completely. Authors purified the receptor with using K562 cells, FC-Siglec-7 fusion protein, diSia-dextran polymer, and identified it as leukosialin (CD43) with MS analysis. Additionally, they demonstrated that the cytotoxicity of NK cells toward K562 cells was suppressed by overexpression of leukosialin in a Siglec-7-dependent manner.