A group from UC San Diego has reported that blood pH, serum Zn concentration, existence of plasma sialoglycoproteins are deeply related to binding formation of High mobility group box 1(HMGB1), which is released from cells into blood with developing sepsis, to leukocyte receptors.
https://www.pnas.org/content/118/10/e2018090118
It was know that HMBG1 works as a chaperone protein controlling gene expression through the interaction with chromatin in healthy cells, and further is passively or actively released from cells into blood in conditions like sepsis, leading to activation of innate immunity, migrating neutrophils to necrotic tissues, and removing such tissues.
http://www.med.osaka-u.ac.jp/introduction/research/endowed/therapy
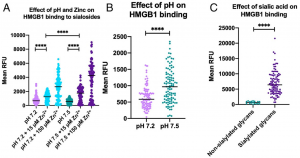
Authors has shown that the binding of HMBG1 to neutrophils is greatly inhibited by the decrease in blood pH and serum Zn concentration, and also the binding is inhibited by preferential binding of HMBG1 to plasma sialoglycoproteins like a lectin binding to sialic acids.
Blood pH is tightly maintained between 7.35 and 7.45 in healthy people. However, it goes down below 7.3 with developing sepsis, and also serum Zn concentration decreases to a level of few µM. In a healthy condition, even if HMGB1 was released from cells into blood, it’s binding to neutrophil receptors could be inhibited by preferential binding to sialoglycoproteins, and thereby no extra inflammation would not be induced. However, with decreasing blood pH and Zn concentration, sialoglycoproteins lose its function as inhibitors, and HMGB1 is able to bind to neutrophil receptors inducing inflammation. That means, a treatment targeting inhibition of HMGB1 with controlling blood pH and the Zn concentration might be also effective in sepsis-related Sequential Organ Failure happening in COVID-19.