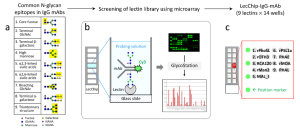
This paper regarding FDA’s dedicated lectin microarray (14-well lectin microarray using 9 kinds of lectins) for the evaluation of mAb drugs (IgG1) has been published.
This paper is related to the Mx blog post on Dec. 8th, 2023.
https://www.tandfonline.com/doi/epdf/10.1080/19420862.2024.2304268?needAccess=true
The 9 kinds of lectins used in this paper and those glycan binding specificities are summarized as follows.
rPhoSL -> core fucose
PHAE -> bisecting GlcNAc
PHAL -> tri/tetra antennary
MAL_I -> α2-3Sia
rPSL1a -> α2-6Sia
RCA120 -> β-Gal
rOTH3 -> terminal GlcNAc
rMan2 -> high mannose
rMOA -> α-Gal
Note that lectins with an “r” at the beginning of their name indicate that they are recombinant.
rOTH3, rMan2 are not official names.
Contact the author of this blog to learn what the official name is.