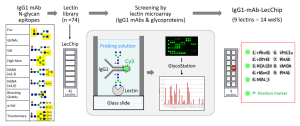
To evaluate glycan epitopes of therapeutic IgG1 mAbs, FDA has developed a new lectin microarray with 9 kinds of lectins, and has demonstrated its effectiveness for high-throughput glycan profiling analysis using GlycoStation Reader 2300 (GSR2300) .
https://www.fda.gov/media/169026/download
2023 FDA Science Forum
The new lectin microarray (IgG1-mAb-LecChip) developed by FDA immobilizes 9 kinds of lectins: rPhosL, rOTH3, RCA120, rMan2, MAL_I, rPSL1a, PHAE, rMOA, and PHEL, and uses a standard 14 wells LecChip format.
Glycan analysis of IgG1 mAbs can be performed using lectin microarrays without creaving glycans, making it possible to perform high-throughput glycan profiling analysis from intact IgG1.
FDA has recommended pharmaceutical companies to use IgG1-mAb-LecChip and GlycoStation to facilitate high-throughput glycan profiling analysis when developing IgG1 mAbs to assess batch-to-batch or biosimilar-to-innovator glycan epitopes.
The figure below shows how IgG1-mAb-LecChip, which was optimized for IgG1 glycan analysis, was developed using GlycoStation and LecChip (n=74 library).
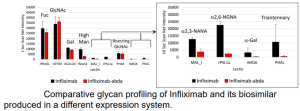
As an example of showing the effectiveness of this technology, the figure below shows the result of evaluating the differences in glycosylation between Infliximab and its biosimilar using IgG1-mAB-LecChip and GSR2300. It can be clearly seen that there are significant differences in the abundance of High Mannose structure, sialic acid modification, and triantennary N-glycans.